Descending of the testes is one of an important processes in the development of male reproductive organs. There is growing evidence from clinical and epidemiological studies in humans and dogs for an increasing incidence of male reproductive disorders including cryptorchidism. In dogs, the incidence of cryptorchidism is variable among breeds, but is most relevant in Chihuahuas and Boxers with 20-30% incidence [
1].
Abnormal estrogen levels can block transabdominal descent of testes and therefore are one of most important factors in this process [
234] and abnormal estrogen action has been implicated as a possible cause for sporadic cryptorchidism in humans [
5] and mice [
678]. Estrogen is mainly produced in the adipose tissue, but its receptors (estrogen receptor α and β; ERα and ERβ) are localized in most cell types in the body including in the testes [
9], which suggests an important role for estrogen in regulating testicular cell function and reproductive events. Deletion of ERα causes infertility in male mice, while the disruption of ERβ does not yield infertile phenotypes, suggesting ERα is essential for male reproduction [
1011] but ERβ has a less important function in the testes [
121314].
It has been reported that ERα is present in the Sertoli cells of multiple species including hystricognath rodents, rats, cats, boars, pigs, and humans [
151617181920212223]. However, there is conflicting data about the expression of ERα in the testes and few studies have been conducted examining the localization of ERα-immunoreactive structures in the testes. In the present study, we investigated the localization of ERα and progesterone receptor (PR) immunoreactivity in the normal testis and cryptorchid testis of a dog.
An 18-month-old German Shepherd with unilateral cryptorchidism was referred to the Seoul National University Veterinary Teaching Hospital, South Korea for elective orchidectomy as explained in a previous study [
24]. The left testis was present within the scrotum but the right testis was not palpable in the scrotum or inguinal area. Laparotomy revealed the cryptorchid testis in the right abdominal region. Both testes were surgically removed.
For histological analysis, both testes were fixed in neutral buffered formalin for 2 days and dehydrated with graded concentrations of alcohol before being embedded in paraffin. Paraffin-embedded tissues were sectioned into 3-µm coronal sections using a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and were mounted onto silane-coated slides (Muto Pure Chemicals Co., Ltd, Tokyo, Japan).
To ensure that the immunohistochemical data were comparable between control and cryptorchid testis, the sections were carefully processed under the same conditions. The sections were hydrated and treated with 0.3% hydrogen peroxide (H2O2) in phosphate-buffered saline (PBS) for 30 min. For antigen retrieval, the sections were placed in 400-mL jars filled with citrate buffer (pH 6.0) and heated in a 2100-retriever (Prestige Medical, Lancashire, UK). After antigen retrieval, slides were allowed to cool at room temperature and were washed in PBS. After washing, the sections were incubated in 10% normal goat serum in PBS for 30 min. They were then incubated with rabbit anti-ERα antibody (1:200; Abcam, Cambridge, UK) or mouse anti-PR antibody (1:200, Abcam) for 48 h at 4℃. They were subsequently exposed to biotinylated goat anti-rabbit IgG, or anti-mouse IgG (diluted 1:200, Vector Laboratories, Inc., Burlingame, CA, USA), and streptavidin peroxidase complex (diluted 1:200, Vector Laboratories). Thereafter, the sections were visualized with 3,3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA) in 0.1 M Tris-HCl buffer (pH 7.4).
Analysis of the regions of interest in the testis was performed using an image analysis system. Images were calibrated into an array of 512×512 pixels corresponding to a tissue area of 140 µm×140 µm (40× primary magnification). Each pixel resolution was 256 gray levels. The intensity of ERα and PR immunoreactivity was evaluated by relative optical density (ROD), which was obtained after transformation of the mean gray level using the formula: ROD=log(256/mean gray level). ROD of background was determined in unlabeled portions and this value was subtracted for correction, yielding high ROD values in the presence of preserved structures and low values after structural loss using ImageJ software v. 1.50 (National Institutes of Health, Bethesda, MD, USA). A ratio of the ROD was calibrated as percentage compared to control.
In the control testis, ERα immunoreactivity was observed in the interstitial space of seminiferous tubules. Based on their location, ERα immunoreactive structures were thought to be Leydig cells of testis (
Figure 1A). In the cryptorchid testis, ERα immunoreactivity was detected in the basal part of seminiferous tubules as well as in the interstitial space of tubules. These cells are judged to be Sertoli cells and Leydig cells, respectively, based on their morphology (
Figure 1B). ERα immunoreactivity in the cryptorchid testis was significantly increased compared to the control testis (
Figure 1C).
 | Figure 1Immunohistochemistry for ERα in the control (A) and cryptorchid (B) testis. Note that ERα immunoreactivity is found in the Leydig cells (arrows) of the control testis, while ERα immunoreactivity is also remarkably detected in the Sertoli cells (arrowheads) of the cryptorchid testis. Scale bar=50 µm. (C) Relative optical density (ROD) of ERα immunoreactivity per section is expressed as percentage of control group (10 sections; *P<0.05, control vs. cryptorchid group). All data are represented as the mean ± SEM.
|
Weak PR immunoreactivity was observed in the spermatids of the control testis, but was not observed in any other structures (
Figure 2A). In the cryptorchid testis, PR immunoreactivity was not detected in any structures (
Figure 2B) and PR immunoreactivity was significantly decreased compared to the control testis (
Figure 2C).
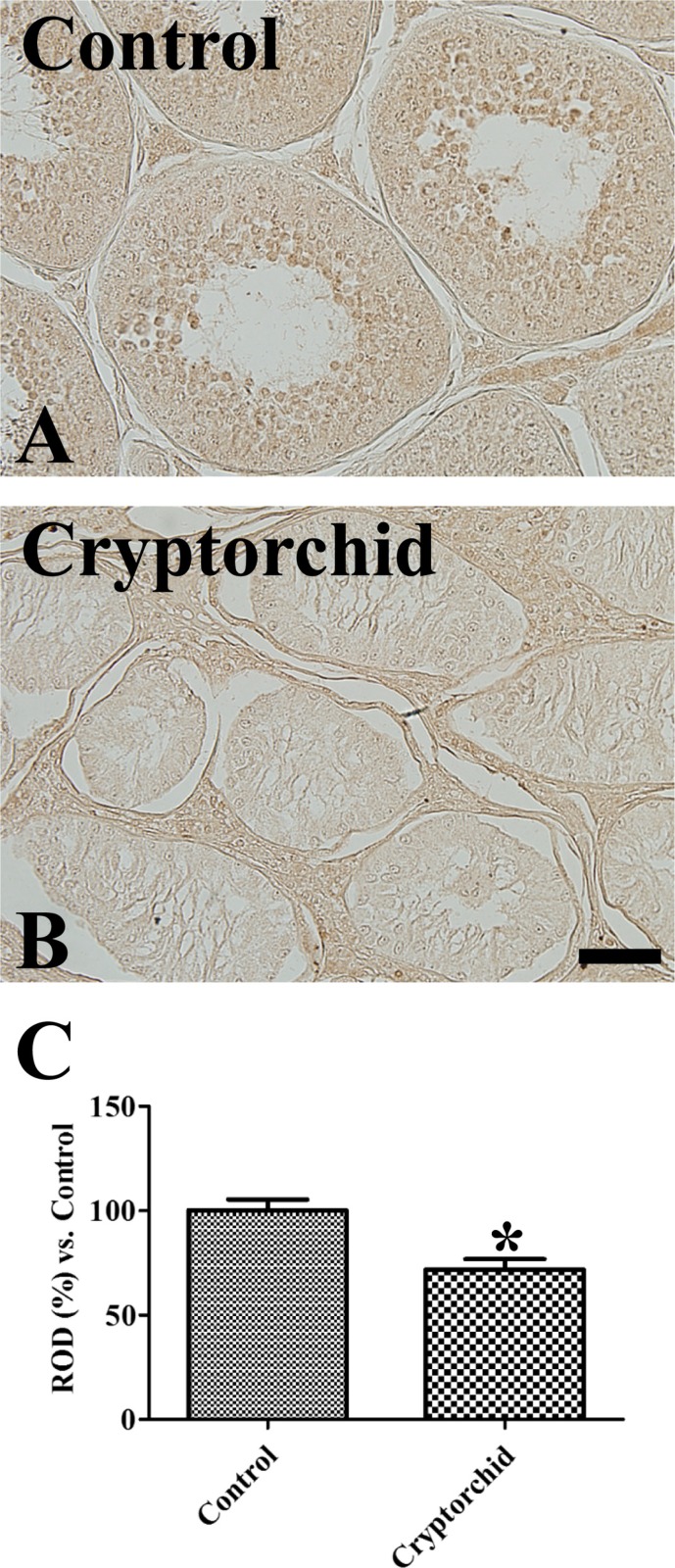 | Figure 2Immunohistochemistry for PR in the control (A) and cryptorchid (B) testis. Note that PR immunoreactivity is only detected in spermatids of control testis. Scale bar=50 µm. (C) Relative optical density (ROD) of PR immunoreactivity per section is expressed as percentage of control group (10 sections; *P<0.05, control vs. cryptorchid group). All data are represented as the mean ± SEM.
|
Spermatogenic failure is the one of the most serious complications in cryptorchidism. In a previous study, we identified morphological abnormalities in cryptorchid testis and increased cell proliferation in cryptorchid Sertoli cells [
24]. In the present study, we observed the localization of ERα and PR in the testis of control and cryptorchid testis belonging to the same dog. ERα immunoreactivity was weakly detected in the Leydig cells of control testis. However, we did not observe any other structures with ERα immunoreactivity. There have been contradictory reports about the expression of ERα in human testes. Some studies showed ERα mRNA [
25] and protein [
26] were not detected in the human testis, nor in primates such as marmoset and macaque [
26]. However, Pelletier and El-Alfy [
27] observed ERα in human Leydig cells and Filipiak et al. [
21] demonstrated that ERα immunostaining was found in the cytoplasm of Sertoli and Leydig cells in humans. Differences we identified in the localization of ERα immunostaining in our study may be closely associated with the expression patterns of ERα in human testes. In the present study, we firstly reported comparison of ERα expressions in intact and cryptorchid testis of unilateral cryptorchid dog.
ERα immunoreactivity was prominently increased in the cryptorchid testis, especially in the Sertoli cells. Several lines of evidence demonstrate that ERα protein is present in the spermatids and Sertoli cells of descended rat testes [
28], as well as in the mesothelial layer of paratesticular tissues of undescended human male testes [
29]. In addition, estradiol levels were higher in the cryptorchid testes than in normal testes as measured by radioimmunological analysis of testicular tissue [
2830]. The increased levels of estradiol may upregulate ERα gene expression in the cryptorchid testis [
2831] and potentiate the effects of estradiol in Sertoli cells.
The role of ERα in the testis has not been fully elucidated. However, transplanted germ cells lacking ERα develop normally in wildtype seminiferous tubules, and can yield offspring by fertilizing oocytes [
3233]. ERα could help provide a favorable environment for gametes to develop and mature [
10], and estrogendependent ERα action is required for germ cell survival, most likely involving the support of Sertoli cell function [
10]. However, in the present study, ectopic expression of ERα in the Sertoli cells of cryptorchid testis may be closely related to the cell proliferation of Sertoli cells, which could progress to Sertoli cell tumors. This result was supported by a previous study that found ERα is frequently expressed in Sertoli-Leydig cell tumors [
34].
In the present study, we did not find any significant differences in PR expression between the control testis and cryptorchid testis. This result was supported by a previous study that found cryptorchidism failed to express PR even though some of these procedures are known to induce progesterone receptor expression in estradiol-target tissues [
35].
In conclusion, unilateral cryptorchidism significantly increases ERα immunoreactivity, not but PR immuno-reactivity, in the Sertoli cells of the cryptorchid testis, and this increase may be associated with proliferation of Sertoli cells in the cryptorchid testis.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download