Abstract
Eosinophilic, polymorphic and pruritic eruption associated with radiotherapy (EPPER) can occur in cancer patients after irradiation. In this study, we characterized the clinical and histopathological features of pig skin that developed widespread polymorphic and pruritic skin lesions following localized 50 Gy gamma-irradiation. The pigs developed pruritus 5-7 weeks after irradiation, and infiltration of the dermis by eosinophils was detected 4-7 weeks after irradiation. The irradiated animals also showed transiently increased numbers of peripheral eosinophils 5-7 weeks after treatment. Irradiation induced desquamation after 2-4 weeks, which and the desquamation gradually resolved after 7 weeks. These pathological changes correspond to those seen in irradiated human skin, indicating that this model could be useful for elucidating the pathogenesis of EPPER and for developing therapeutic and prophylactic methods.
Radiation-induced skin damage can lead to various skin eruptions, most frequently erythema multiforme [1], generalized papuloerythematous rashes [2], widespread and nonspecific skin eruptions, or bullous pemphigoid [3]. Previous studies found that a number of cancer patients developed an intense, widespread, polymorphic, and pruritic eruption lasting several weeks or months, usually while undergoing radiotherapy [45]. The clinical, histopathological, and immunopathological features of this condition in cancer patients undergoing radiotherapy have been described, albeit incompletely. Due to its unique presentation, the denomination "eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy" (EPPER) has been suggested [6]. This eruption shows some similarities to other diseases in which eosinophils play an important role. In this study, we attempted to better characterize EPPER with respect to its epidemiological, clinical, and histological features. In addition, although a number of clinical studies of cancer patients have reported pruritus following radiotherapy, it could not be proven that radiation caused this condition [4578], and we therefore aimed to develop a model that would allow this relationship to be further evaluated.
Rodents, such as the rat and mouse, are frequently used in radiation-induced skin injury studies as they are inexpensive and easy to handle. Despite these advantages, rodents differ from humans in a number of anatomical and physiological ways. For example, these mammals have a dense layer of body hair and a thin epidermis and dermis, and, more significantly, they heal primarily through wound contraction as opposed to re-epithelialization. Humans and pigs heal through physiologically similar processes. Additionally, the pig's overall physiology is close to human physiology, with most key organ systems being similar in anatomy and function [9]. Anatomically and physiologically, pig skin is more similar to human skin, and both have a thick epidermis. The pig has therefore been widely used for modeling radiation-induced skin injuries.
In this study, we performed focal irradiation of porcine dorsal skin in order to model the changes that occur in human skin after radiotherapy
Six male minipigs (mean weight, 19 kg; range 18-20 kg) were obtained from PWG Genetics (Seoul, Korea). The minipigs were fed a standard animal diet. All animal experiments followed a protocol approved by the Institutional Animal Care and Use Committee of the Korea Institute of Radiological and Medical Sciences (KIRAMS).
In order to observe the effects of gamma irradiation on minipig skin, the flanks of the animals were irradiated. For all procedures, the animals were anesthetized with tiletamine/zolazepam (Zoletil 50®, Virak Korea, Seoul, Korea) and medetomidine (Domitor®, Pfizer Animal Health Korea, Seoul, Korea). Three to 4 days prior to irradiation the hair was clipped from the areas to be exposed and the position of the fields was marked and tattooed with India ink. The fields were gamma irradiated with 50 Gy using a 60Co gamma-ray irradiation unit (Theratron 780, AECL, Ontario, Canada) at a dose-rate of 130.1 cGy/min (field size: 5 cm, Source skin distance: 80 cm, depth: 1 cm [bolus 1 cm]); the area of flank skin available allowed a 50 Gy irradiation dose to be given to each pig (Figure 1A). At the time of irradiation, 3 skin biopsies were taken and pinned to cork to maintain the original size. A 5-mm punch biopsy was obtained under anesthesia from the irradiated skin area and the corresponding area of the healthy skin. Samples of the biopsy were processed for embedding in paraffin wax after fixation in 10% buffered formalin.
The pigs were carefully evaluated during the 8 weeks following irradiation, and the radiation-induced acute skin reactions were measured using a clinical scoring system, as follows: 0, normal; 10-30, slight swelling, edema, and erythema; 40-60, dry desquamation and mild early moist desquamation changes with partial loss of the epidermal basal cells, dryness, itching and scaling; 70-90, severe moist desquamation and some necrosis with complete destruction of the basal cell layer, blistering and serous drainage; and 100, severe ulceration and loss of dermis [10].
Pruritic behavior in pigs is more complex than in humans, and includes limb movements, licking, biting and rubbing. Cameras were attached to a wall 24 hours before recording started to allow the pigs to habituate to their presence. The following scoring system was used to measure pruritus: 0, no signs of skin irritation; 1, very mild itching/only occasional episodes; 2, mild itching, somewhat more frequent; 3, moderate itching, regular episodes; 4, severe itching, prolonged episodes; and 5, extremely severe itching, almost continuous [11].
For examination by light microscopy, we used tissue samples from the biopsy of each skin field that had been fixed in 4% buffered paraformaldehyde, dehydrated in graded ethanol, and embedded in paraffin. Thereafter, 4 µm thick sections of tissue were cut on a rotary microtome (Leica Ultramicrotome) and mounted on clean glass slides. Sections were cleared, hydrated, and stained with hematoxylin and eosin for histological evaluation of skin injury, epidermal thickness, and basal cell density, respectively, according to standard protocols. The slides were coded to prevent observer bias during evaluation. All tissue sections were microscopically examined in order to evaluate histopathological changes. Skin samples were photographed using a digital camera mounted on a microscope (Leica DM IRBE, Leica Microsystems GmbH, Wetzlar, Germany), and the images were analyzed using image analysis software (Leica QWin, Leica Microsystems, Wetzlar, Germany). The longest rete ridge on each slide was measured from the bottom of the basal layer to the bottom of the stratum corneum, avoiding areas where the inclusion seemed to be oblique. The means were calculated from each slide. Cell density in the basal layer was determined by counting cells along a minimum of 5 mm of basement membrane, and the results were expressed as the count per mm of basement membrane. Degenerate cells, i.e. those showing pyknosis or shrinkage necrosis, were excluded from these calculations.
Blood samples were collected through an ear vein into sample tubes containing ethylenediaminetetraacetic acid at different time points. Peripheral eosinophils were automatically counted using a HemaVet System (Drew Scientific Inc., UK).
The data is reported as the mean±SEM, and was analyzed using one-way analysis of variance (ANOVA) followed by a Student-Newman-Keuls post hoc test for multiple comparisons. In all cases, a P value<0.05 was considered significant.
The time-dependent gross changes in the skin are summarized in Figure 1D. A radiation dose of 50 Gy damaged the epithelial layer, initially resulting in erythema. This was followed by increased pigmentation, epilation, and desquamation, and 2 weeks after irradiation the skin usually showed desquamation associated with bright red erythema. This reaction became gradually more severe, and after 4 weeks moist desquamation persisted, tissue breakdown extended into the dermis, and the area became infected. This was termed ulceration, and healing from this type of reaction resulted in tissue scarring. After 5 weeks the most severe reaction was damage of the epithelial layer preceded by a dusky red or mauve erythema. Some fields that were irradiated with a 50 Gy dose developed moist desquamation after irradiation (Figure 1C). After 5 weeks, the clinicopathological changes began to diminish, and the treated skin had recovered and appeared similar to the untreated skin 7-9 weeks post-irradiation (Figure 1D).
Minimal pruritus was found in the sham irradiated pigs. Irradiation increased itching behavior in a time dependent manner (Figure 1E), 1-5 weeks after treatment. The itching was most severe 5 weeks after irradiation, and could not even be prevented in the consulting room. In some animals itching also occurred at night, and when eating, playing, and exercising, even when an attempt was made to distract the animal animal. Itching behavior decreased gradually to that observed in the sham irradiated pigs 9 weeks after treatment.
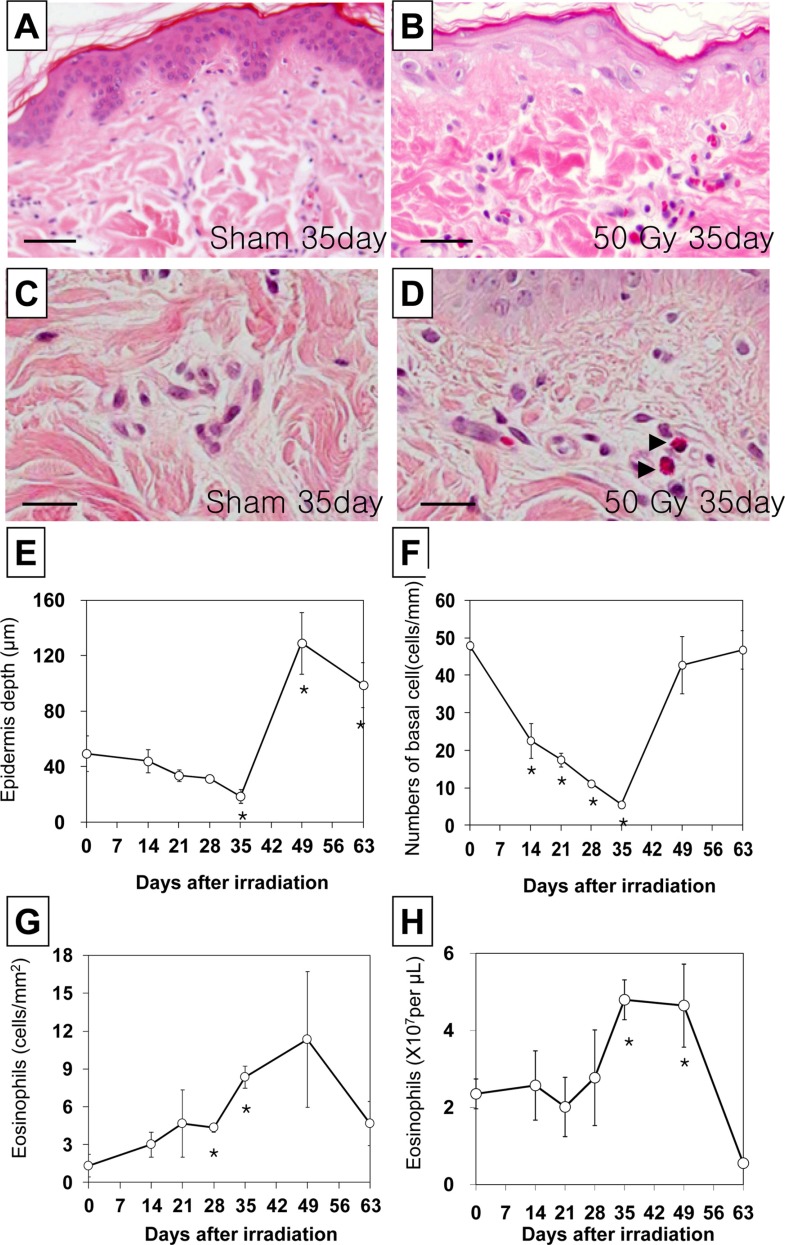
Biopsy specimens from all of the irradiated pigs showed an inflammatory pattern composed mainly of superficial and deep perivascular infiltrates of variable severity, in most cases confined to the dermis (Figure 2B). Changes in the epidermal thickness and basal cell density were correlated with clinical changes (Figure 2E,F). The epidermal thickness of the sham irradiated and irradiated skin are shown in Figure 2E. The thickness of the epidermis gradually decreased until 5 weeks after irradiation (n=3, P<0.05 vs control; Figure 2E), but then sharply increased compared to that of the sham control 7-9 weeks after treatment (n=3, P<0.05 vs. control; Figure 2E), and thereafter gradually returned to a value close to that of the control skin.
The density of basal cells in the epidermis in sham irradiated and 50 Gy irradiated skin is shown in Figure 2F. The density of basal cells in the epidermis gradually decreased between 2 and 5 weeks following irradiation (n=3, P<0.05 vs. control; Figure 2F), and then increased to the sham control level 6-8 weeks after irradiation (Figure 2F).
The levels of skin associated tissue eosinophils and peripheral eosinophils were evaluated semiquantitatively after focal irradiation. Eosinophils began to accumulate in the dermis of the irradiated pigs (Figure 2D). The number of skin (Figure 2G) and peripheral (Figure 2H) eosinophils peaked within 5-7 weeks post irradiation, and then returned to the sham control level.
Several clinical studies have reported that EPPER is a side effect of radiotherapy in cancer patients. A number of factors may protect against, or increase the risk of EPPER, including the type of cancer and its treatment, and individual patient factors. The existence of EPPER has become generally accepted, although many details remain controversial or obscure. We characterized the epidemiological, clinical, and histopathological features of pig skin that developed widespread polymorphic and pruritic skin lesions following a 50 Gy radiation dose, and showed that these changes were similar to those seen in irradiated human skin.
EPPER is relatively rare in cancer patients after radiotherapy, although it occurred in all of the irradiated pigs. Eosinophils play a critical role in late-phase reaction allergic inflammatory responses, although the factors responsible for selective tissue eosinophilia are currently ill-defined. A few closely related dermatoses characterized by sustained eosinophilia of an undetermined cause associated with hematologic, cardiac, and neurologic abnormalities have been collectively described as "idiopathic hypereosinophilic syndrome", and in some patients response and survival improved after chemotherapy [1213]. The change in the number of eosinophils in the peripheral blood after exposure to radiation appears to vary with the species and exposure conditions. Although irradiation of most animal species depresses the eosinophil count, it causes eosinophilia in man [14].
Although we failed to determine the mechanistic basis of EPPER in porcine skin, we were able to show that the clinical changes after irradiation were similar to those seen in the skin of human patients. We also found that eosinophils infiltrated the irradiated skin and induced pruritus. This suggests that the pig may be a good model for studying EPPER.
Acknowledgment
This study was supported by the project titled 'Development of therapeutic improvement on acute radiation syndrome (50581-2013)' of Ministry of Science, ICT and Future Planning (MSIP), Korean government.
References
1. Davis J, Pack GT. Erythema multiforme following deep x-ray therapy. AMA Arch Derm Syphilol. 1952; 66(1):41–48. PMID: 14932502.

2. Dedick AP Jr, Whelan VM. Generalized skin reaction following deep x-ray therapy. Radiology. 1959; 72(5):751–753. PMID: 13658411.

3. Duschet P, Schwarz T, Gschnait F. Bullous pemphigoid after radiation therapy. J Am Acad Dermatol. 1988; 18(2):441–444. PMID: 2963841.

4. García-Donoso C, Tardío JC, Arias D, Romero A, Borbujo JM. Eosinophilic, polymorphic and pruritic eruption associated with radiotherapy (EPPER) in two patients with breast tumour. J Eur Acad Dermatol Venereol. 2007; 21(8):1102–1104. PMID: 17714133.

5. Lee DJ, Kim YC. A pustular form of eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy. J Am Acad Dermatol. 2011; 65(2):e51–e53. PMID: 21763551.

6. Rueda RA, Valencia IC, Covelli C, Escobar C, Alzate A, Saldarriaga B, Sanclemente G, Blank A, Falabella R. Eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy. Arch Dermatol. 1999; 135(7):804–810. PMID: 10411155.

7. Lam Cham Kee HX, Charra-Brunaud C, Cuny JF, Reigneau M, Vogin G, Peiffert D. [Case report of EPPER Syndrome (eosinophilic polymorphic pruritic eruption associated with radiotherapy) in a patient treated against endometrial cancer]. Cancer Radiother. 2013; 17(1):54–57. PMID: 23291008.
8. Shimamoto N, Shirase T, Yoshikawa Y. Vesicular form of eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy confined to the irradiated area. J Dermatol. 2013; 40(3):228–229. PMID: 23289628.

9. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001; 9(2):66–76. PMID: 11350644.

10. Kim JS, Rhim KJ, Jang WS, Lee SJ, Son Y, Lee SS, Park S, Lim SM. β-irradiation (1Ho patch)-induced skin injury in mini-pigs: effects on NF-κB and COX-2 expression in the skin. J Vet Sci. 2015; 16(1):1–9. PMID: 24962420.
11. Hill PB, Lau P, Rybnicek J. Development of an owner-assessed scale to measure the severity of pruritus in dogs. Vet Dermatol. 2007; 18(5):301–308. PMID: 17845617.

12. Spry CJ, Davies J, Tai PC, Olsen EG, Oakley CM, Goodwin JF. Clinical features of fifteen patients with the hypereosinophilic syndrome. Q J Med. 1983; 52(205):1–22. PMID: 6878618.
13. van den Hoogenband HM. Skin lesions as the first manifestation of the hypereosinophilic syndrome. Clin Exp Dermatol. 1982; 7(3):267–271. PMID: 7105478.

14. Kurohara SS, Hempelmann LH, Englander CL, Fuller LM, Rubin P. Eosinophilia after exposure to ionizing radiation. Radiat Res. 1964; 23(3):357–368. PMID: 14223308.

Figure 1
Clinical changes in the skin of a minipig following a 50 Gy irradiation. A. Focal gamma irradiation (50 Gy) was applied to dorsal skin of mini-pigs. B. Sham (0 Gy) control and C. 35 days after irradiation (50 Gy). D. Time-dependent changes in the skin following irradiation. E. Time-dependent development of pruritus following irradiation. The data is reported as the mean±SEM. *P<0.05 vs. sham controls.
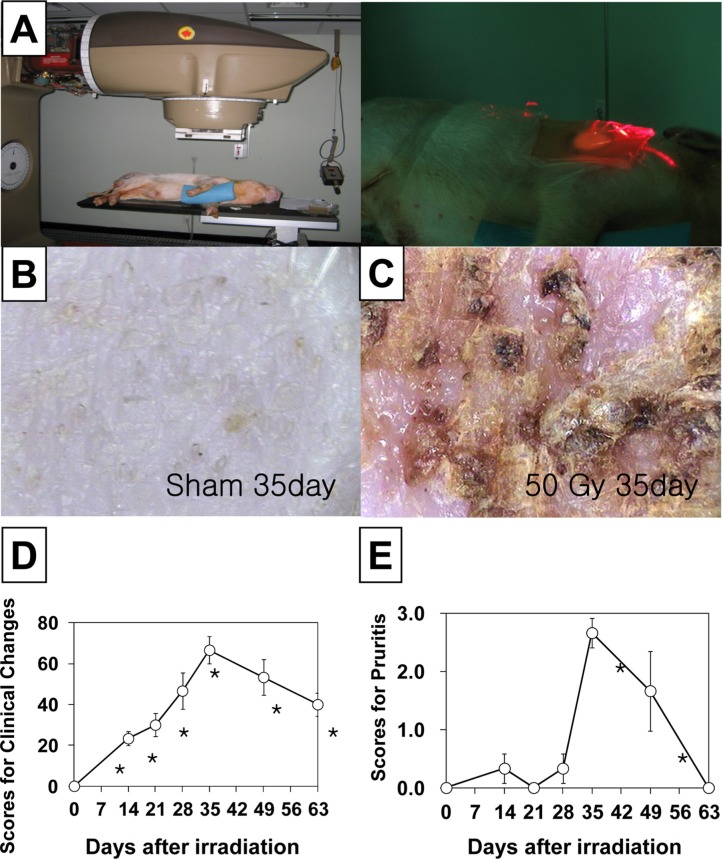
Figure 2
Histological changes in the skin in a hematoxylin and eosin stained section of minipig skin following a 50 Gy irradiation. A and C Skin section from the sham group representative of normal morphology. B and D Skin section from pigs 35 days after irradiation. Basal cell density and epidermal thickness decreased, and eosinophil infiltration increased in the skin 35 days after irradiation. A and B magnification, ×200. Scale bars in A and B: 50 µm. C and D magnification, ×1000. Scale bars in C and D: 20 µm. Time-dependent changes in basal cell density (E), epidermal thickness (F), and the number of dermal (G) and peripheral (H) eosinophils in minipig skin following acute irradiation.. The data is reported as the mean±SEM. *P<0.05 vs. sham controls.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download