Abstract
The aim of this study is to assess the effects of different glucocorticoid administration protocols on biomechanical properties of the first lumbar vertebral body in rats. We divided 40 male rats into the following groups: control, dexamethasone (7 mg/week), dexamethasone (0.7 mg/week), methylprednisolone (7 mg/kg/week), methylprednisolone (5 mg/kg twice weekly), dexamethasone (7 mg/kg three times per week), dexamethasone (0.7 mg/kg three times per week, and low-level laser treated rats. Lumbar vertebrae in rats were exposed to the pulsed laser. We conducted a biomechanical test to examine the mechanical properties of vertebral body in rats' lumbar bone. Supraphysiologic glucocorticoid administration protocols did not impair the biomechanical properties of rats' vertebral bodies compared to control and laser-treated rats. Supraphysiologic glucocorticoid administration caused an anabolic effect on the vertebral bodies.
Osteoporosis (OP) is a problem which causes bone lost and fractures which leads to severe pain, deformity; the secondary complications, and eventually possibility of death [1]. Glucocorticoids (GCs) are potent anti-inflammatory and immunosuppressive drugs. Synthetic GCs have been widely used for many decades to treat various problems including: autoimmune, pulmonary, periodontal, and gastrointestinal disorders [2]. In the other hand, GC-induced osteoporosis (GIOP) in which constitutes the most frequent secondary OP. To have better preventions and treatments for debilitating fractures which occurs predominately in the spine, understanding the pathology of GC which induces bone lost has a crucial importance [3].
According to the National Health and Nutrition Examination (NHANES) from 1999-2008, the prevalence of GC and anti-osteoporotic medications were approximately 1.2% in the United States in which the number is much higher than other countries [4].
To investigate the pathogenesis of OP, different animal models have been used; also facilitated preclinical testing, and new treatment options such as anti-resorptive drugs [5]. In the animal study, histophorphometric parameters and biochemical markers indicate a decrease in bone formation and minimal changes in bone resorption. These parameters are less important with regards to OP-associated fractures and investigations in orthopedic surgery. Histological and biomechanical studies do not give direct information about the mechanical strength in bone. The most important outcome in bone fracture which occurs by a simple trauma is reduction in mechanical strength [6]. Although bone densitometry is often used as a surrogate to evaluate bone fragility, direct biomechanical testing surly provides more information about mechanical integrity of bone [7]. Small animal models, in particular GIOP rat models are reported to be relatively resistant to corticosteroid-induced bone damage [8910]. However, many studies in vivo have shown GC administration to rats both histologically and histomorphometrically inhibits bone formation in the trabecular bone; particularly in the vertebrae [111213141516171819202122]. In the other hand, it would reduce bone strength [12152122] and bone mineral density (BMD) as shown by BMD and other radiologic imaging techniques [1113161921232425]. In addition, It would cause a decrease in biomarkers in the serum [131516172425]. Eventual, GCs cause decrease in the molecular elements of bones as shown by the molecular biology techniques [141718202225].
The term low-level laser therapy (LLLT) is broadly applied to the therapeutic effects of low-level lasers. Professor Mester was the first researcher who reported clinical application of LLLT in 1968. Previous studies have shown the positive effects of LLLT both on intact bone and the bone healing process [26]. LLLT also acts as a proposed anabolic therapeutic agent on bony tissues.
The most common fractures are those of the vertebrae, proximal femur, and distal forearm (wrist) [27]. Approximately 80 per cent of the total skeleton is composed of cortical bone, the rest is trabecular bone. Certain regions of the skeleton are rich in trabecular bone; including vertebrae, femoral neck, and distal radius [28].
The aim of this study was to assess the effects of GCs (methylprednisolone and dexamethasone) administration protocols on the biomechanical properties in the first lumbar vertebral body. The Analyses indicate bending stiffness (Young's modulus of elasticity), maximum force, stress high load, and energy absorption up to maximum force as assessed by a biomechanical compression test. The data were compared on biomechanical properties of the vertebral body in GC-treated rats; with healthy control and healthy laser-treated rats.
In this study, the total of 40 male Wistar rats 4.5 months were housed in standard rat cages with a 12 h light/dark schedule. Rats were provided water ad libitum. All procedures were approved by the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (protocol no 1391-1-115-1092). The rats' weights were measured weekly and the volume of drugs administered was calculated based on the most recent body weight.
The rats were randomly allocated into eight groups in which each group included 5 rats [2930]. The groups received the following drugs and laser treatment: (1) vehicle (intramuscular injection of 100 µL distilled water, twice a week); (2) dexamethasone (7 mg/kg/week, 5 injections over 5 weeks; Dex 7 mg/kg, I) (18); (3) dexamethasone (0.7 mg/kg/week, 5 injections over 5 weeks; Dex 0.7 mg/kg, I); (4) methylprednisolone (7 mg/kg/week, 5 injections over 5 weeks; Met 7 mg/kg, I) [11]; (5) methyprednisolone (5 mg/kg given five times in the first week [19] and twice weekly thereafter). The protocol was revised after observing a sever weight lost in the rats for the total of seventeen injection in seven weeks; Met 5 mg/kg, II); (6) dexamethasone (7 mg/kg given three times per week for the total of 16 injections over 7 weeks; Dex 7 mg/kg, III); (7) dexamethasone (0.7 mg/kg give three times per week for the total of 21 injections over seven weeks (Dex 0.7 mg/kg, III).
We observed sever weight lost in the entire population who received GC in the second, fourth, fifth, and sixth groups. In the second and fourth groups two rats died from each groups. In the fifth and sixth groups, three rats died. The deaths were probably the result of unexpected high weight lost that might have been caused by the depot effect of GC administration [12]. The dead rats were replaced.
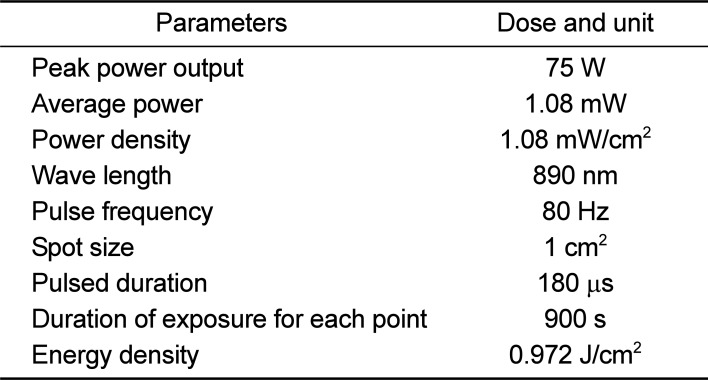
Pulsed wave (PW) LLLT was performed on the spinal processes in T12, L1, L2 , and L3 vertebrae. The laser pen was held perpendicular to the target tissue at the distance of <1 cm with an infrared diode laser [MUSTANG 2000 with LO7 pen (radiating head), Technica Co., Moscow, Russia]. Specifications of the laser are shown in Table 1.
In our study, the surface area of the target tissue (vertebral body of T11 thorough L3 in the vertebral column) was larger than the pen's spot size; therefore, we used sequential treatments to ensure that every unit area received the same dosage [31]. We performed LLLT on four distinct regions by four shootings. During LLLT the animals were sedated by administration of ketamine hydrochloride (25 mg/kg body weight); injected intramuscularly along with diazepam (25 mg/kg body weight). LLLT was performed one a day; six days per week, for the total of three weeks.
By the end of treatment, all the animals were sacrificed by injecting overdose anesthetized drugs that followed by cervical dislocation. L1 vertebral bones of all rats were extracted and kept moist throughout the testing procedure. We removed nearly all soft tissues attached to the bones. The vertebral bodies were subjected to a compressive test on the material testing device (Zwick/Roell, Germany) until fracture occurred; we defined breaking point when bone broke into numerous pieces. All bones were oriented similarly in the testing machine and the surface areas of bones were also calculated by multiplying the breadth by itself. Bones were mounted into a machine, and a press head was activated to compress the center of the vertebral body until fracture occurred. The compressive loading speed was 0.08 mm/s for all tests. Specimens were loaded uniaxially; therefore, the fracture and complete load-deformation curve could be recorded. From the load-deformation curve, the following biomechanical properties were automatically calculated: bending stiffness (Young's modulus of elasticity; N/mm2), maximum force (N), stress high load (N/mm2), and energy absorption up to maximum force (N mm) (Figure 1).
Bending stiffness is the slope of the linear portion of the load-deformation curve. Maximum force is the force needed to break a bone microscopically. The stress high load was calculated by dividing the means of maximum force value by the surface area (mm2) of bones. Energy absorption up to the maximum force is the amount of energy absorbed by a bone until it breaks microscopically at the point of maximum force [32].
All data are expressed by mean±standard error of mean (SEM). The overall differences of all biomechanical parameters with the exception of bending stiffness were analyzed by using one way analysis variance. Group comparisons were obtained by applying the least significant difference (LSD) method in the analysis. The overall differences of bending stiffness were analyzed by using the Kruskal-Wallis test. The group comparisons were obtained by applying the Mann Whitney U test method in the analysis. The P-value less than 0.05 was significant for ANOVA, LSD, and the Kruskal-Wallis test. The P-value below 0.007 were considered significant for the Mann Whitney U test [2930].
Figure 2 shows the rats' body weights. Biomechanical examination results are shown in Figures 3, 4, 5, 6. According to the results GC administration protocols did not impair the biomechanical properties of the rats' vertebral bodies. The biomechanical properties of GC-treated rats were comparable with those of control and LLLT-treated rats.
As seen in Figure 2 there were significant increases in bending stiffness of GC- treated rats from groups 4 (LSD test, P=0.01), 6 (LSD test, P=0.004), and 7 (LSD test, P=0.02) compared to control rats (ANOVA test, P=0.007).
We observed significant increases in maximum force of GC treated-rats from groups 2 (LSD test, P=0.005), 4 (LSD test, P=0.017), and 6 (LSD test, P=0.035) compared to control rats (ANOVA test, P=0.006). GC treated-rats from groups 2 showed significant increases in maximum force compared to LLLT treated-rats (P=0.037).
We established different rat models based on previously published rat models for GC administration. The intent was to investigate GC side effects on the biomechanical properties of rats' first lumbar vertebrae. Although weight lost was observed in the entire population which received GC; it seemed more severe in the fifth and sixth groups. Rats from the fifth group received 17 injections of 5 mg/kg methylprednisolone for seven weeks; and rats from the sixth group received 16 injections of 7 mg/kg dexamethasone for seven weeks. The mortality rate for GC-treated rats was 21%. The biomechanical properties of the vertebral body in GC-treated rats were comparable to those in the control and LLLT rats. Previous studies have shown the positive effects of LLLT both on intact bones and bones with healing process (26). It can act as a proposed anabolic therapeutic agent on bones [26].
According to our analysis, these findings might be due to the anabolic effects of GC administration in trabecular bone. The result of biomechanical examinations in GC-treated rats were accompanied by weight lost and high rate of mortality. These data were markedly distinct from findings in patients with supraphysiologic GC administration who showed bone lost. Supraphysiologic doses of GCs also caused weight gain in patients [33].
As reported in the current study, the anabolic effects of GC administration on the trabecular bone was consistent with previous studies [8910].
Treatment with dexamethasone for 13 days in male rats caused no histomorphometrical differences and bone density of vertebral trabecular. Mean trabecular bone density in tibial metaphysis increased because of dexamethasone, presumably due to osteoclast inhibition. Dexamethasone-treated rats lost weight [8]. Li et al. reported treatment of rats with corticosteroid increased the trabecular mass in the lumbar vertebrae [9]. Prednisolone treatment over four weeks period in rats significantly increased BMD and trabecular bone volume compared to the non-prednisolone treated control group. Mechanical strength testing in trabecular bone of distal femur reflected changes in BMD and trabecular bone volume. Shen et al. concluded, unlike the effects observed in humans treated with GCs, treatment in rats with prednisolone did not cause bone lost; in contrast, it caused a protective effect on the skeleton through the inhibition of bone resorption [26]. Twenty-eight days treatment with corticosterone in transgenic mouse models significantly increased the pericortical cross-sectional zone in long bones. Vertebral cortical thickness and surface area were reduced in corticosterone-treated mice. Transgenic mice were partly protected from effects of exogenous corticosterone, both at the cellular and structural levels. The corticosterone dosage used in this study, caused trabecular bone remains largely unaffected. Henneicke et al. concluded that increase in tibial pericortical cross-sectional area and changes in the pericortical circumference results an anabolic bone response on GC treatment at this site [34].
According to our study GC administration increased biomechanical properties of vertebral body; in contrast to other studies state, GC administration decreased biomechanical properties of vertebral body [12152122]. Ortoft et al. examined the effects of GC on vertebral bone, and the effect of growth hormone on vertebral bone in young growing animals who received GC injections. According to their study, five groups of 14-weeks- old female rats were treated for 80 days as follows: (1) saline, (2) prednisolone (5 mg/kg/day), (3) growth hormone (5 mg/kg/day), (4) prednisolone and growth hormone, and (5) food restriction. Ortoft et al. found that administration of growth hormone increased body weight, vertebral height, cross-sectional area, and volume. The compressive strength of L4-vertebral body was also increased because of an increase in cancellous bone volume; in addition, increased the area of cortical bone that surrounded the vertebral body. GC administration decreased body weight, height, and volume of the intact vertebrae. No effect of GC administration on mechanical strength in L4 body could be detected. They showed no effect of growth hormone in vertebral bone; when administered to animals who additionally received GC injections. GC administration decreased longitudinal growth of the vertebrae and cortical bone mass; however, it did not affect cancellous bone mass in the vertebral body. Despite this, administration of GC with a depot effect totally inhibited growth hormone on vertebral bone [12].
In the study by Hulley et al., OP was induced in 3.5-month-old rats. The rats received 3.5 mg/kg of methylprednisolone per day for nine weeks. Rats were treated with steroid solo or in combination with 0.5 mg/mL sodium orthovanadate (protein tyrosine phosphatases, (PTP)). The bones treated with steroid were significantly osteopenic and physically weaker than the control group. Concomitant treatment with vanadate largely prevented the densitometric, histologic, and physical abnormalities induced by prednisolone. Hulley et al. concluded that PTPs were central to the negative regulation of osteoblast proliferation by GC; and recommended that PTP inhibitors molecules such as sodium orthovanadate should be considered as novel anabolic agents for the treatment of steroid-induced OP [15].
To distinguish the difference in the effect of GC on osteoclasts, osteoblasts, or osteocytes, Weinstein et al. administered GC solo or in combination with the antagonist osteoprotegerin (OPG) and the fragment crystallizable region of Ig heavy chains (OPG-Fc) . They determined that the suppressive effect of GC on spinal bone mineral density, cortical thickness, and strength was prevented by OPG-Fc. OPG-Fc, with or without GC, profoundly reduced osteoclasts, osteoblasts, and bone formation. Unexpectedly, OPG-Fc prevented GC-induced increase in osteocyte apoptosis, and reduction in solute transport from the systemic circulation to the osteocyte-lacunar-canalicular network. Weinstein et al. concluded that at least part of the OPG-induced preservation of bone strength was attributed to the maintenance of osteocyte viability and the lacunar-canalicular network [21].
Salvianolic acid B is a polyphenolic compound from a Chinese herbal medicine, Salvia miltiorrhiza Bunge. Cui et al. evaluated the effects of salvianolic acid B on osteoblast bone formation, angiogenesis, and adipogenesis-associated GIOP. Bone loss in GC-treated rats were confirmed by significantly decreased BMD, bone strength, cancellous bone mass architecture, osteoblast distribution, bone formation, marrow microvessel density diameter along with down-regulation of marrow Bone Morphogenic Protein (BMP) expression and increased adipogenesis. Daily treatment with salvianolic acid B for twelve weeks in GC-treated male rats prevented GC-induced cancellous bone lost; in addition, increased adipogenesis while increasing the rate of cancellous bone formation by capillary dilation, the local microcirculation was improved. Cui et al. concluded salvianolic acid B prevented bone lost in GC-treated rats through stimulation of osteogenesis, bone marrow angiogenesis, and inhibition of adipogenesis [22].
The cellular mechanisms underlying the anabolic effects of GC administration on the vertebral body observed in the current study remain unclear.
Further elucidation in our findings may shed light over the physiology of trabecular bone formation and how GC affects this process in rats.
GCs have potent clinical effects when used at pharmacological doses. However, their clinical benefits are often marred by serious adverse effects. A clear understanding of the molecular and cellular mechanisms underlying anabolic effects of GC could direct scientist toward new medications with anabolic characteristic, and thus could have a positive therapeutic application. This is clinical relevance of our work.
We have concluded that in marked contrast to the findings in human and in other animals, supraphysiologic administration of GC caused severe weight lost and a considerable mortality rate in rats. It is caused by increased biomechanical properties' values of the rats' vertebrae, which was comparable with those in the control and LLLT groups. This finding might reflect anabolic effect of GC administration on the trabecular bony tissue in rats' vertebral bodies.
Acknowledgment
We wish to extend our sincere thanks to the late Mrs. Jamileh Rezaei. The present article is financially supported by "Research Department of the School of Medicine" (Grant No. 1391-1-115-1092) at Shahid Behesti University of Medical Sciences, Tehran, Iran.
References
1. Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B. Mortality after osteoporotic fractures. Osteoporos Int. 2004; 15(1):38–42. PMID: 14593451.

2. Kim HJ. New understanding of glucocorticoid action in bone cells. BMB Rep. 2010; 43(8):524–529. PMID: 20797313.

3. Popp AW, Isenegger J, Buergi EM, Buergi U, Lippuner K. Glucocorticosteroid-induced spinal osteoporosis: scientific update on pathophysiology and treatment. Eur Spine J. 2006; 15(7):1035–1049. PMID: 16474946.

4. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013; 65(2):294–298. PMID: 22807233.

5. Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporos Int. 2005; 16(2 S):S129–S138. PMID: 15750681.

6. Peng Z, Tuukkanen J, Zhang H, Jämsä T, Väänänen HK. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone. 1994; 15(5):523–532. PMID: 7980963.

7. Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993; 14(4):595–608. PMID: 8274302.

8. Binz K, Schmid C, Bouillon R, Froesch ER, Jürgensen K, Hunziker EB. Interactions of insulin-like growth factor I with dexamethasone on trabecular bone density and mineral metabolism in rats. Eur J Endocrinol. 1994; 130(4):387–393. PMID: 8162170.
9. Li M, Shen Y, Halloran BP, Baumann BD, Miller K, Wronski TJ. Skeletal response to corticosteroid deficiency and excess in growing male rats. Bone. 1996; 19(2):81–88. PMID: 8853849.

10. Shen V, Birchman R, Liang XG, Wu DD, Lindsay R, Dempster DW. Prednisolone alone, or in combination with estrogen or dietary calcium deficiency or immobilization, inhibits bone formation but does not induce bone loss in mature rats. Bone. 1997; 21(4):345–351. PMID: 9315338.

11. Wimalawansa SJ, Chapa MT, Yallampalli C, Zhang R, Simmons DJ. Prevention of corticosteroid-induced bone loss with nitric oxide donor nitroglycerin in male rats. Bone. 1997; 21(3):275–280. PMID: 9276093.

12. Ortoft G, Oxlund H, Andreassen TT. Administration of a glucocorticoid with depot effect counteracts the stimulating effect of growth hormone on cancellous and cortical bone of the vertebral body in rats. Calcif Tissue Int. 1998; 63(1):14–21. PMID: 9632841.
13. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998; 102(2):274–282. PMID: 9664068.

14. Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002; 109(8):1041–1048. PMID: 11956241.

15. Hulley PA, Conradie MM, Langeveldt CR, Hough FS. Glucocorticoid-induced osteoporosis in the rat is prevented by the tyrosine phosphatase inhibitor, sodium orthovanadate. Bone. 2002; 31(1):220–229. PMID: 12110438.

16. McLaughlin F, Mackintosh J, Hayes BP, McLaren A, Uings IJ, Salmon P, Humphreys J, Meldrum E, Farrow SN. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone. 2002; 30(6):924–930. PMID: 12052464.

17. Conradie MM, de Wet H, Kotze DD, Burrin JM, Hough FS, Hulley PA. Vanadate prevents glucocorticoid-induced apoptosis of osteoblasts in vitro and osteocytes in vivo. J Endocrinol. 2007; 195(2):229–240. PMID: 17951534.
18. Lucinda LM, Vieira BJ, Oliveira TT, Sá RC, Peters VM, Reis JE, Guerra MO. Evidences of osteoporosis improvement in Wistar rats treated with Ginkgo biloba extract: a histomorphometric study of mandible and femur. Fitoterapia. 2010; 81(8):982–987. PMID: 20600689.

19. Sun P, Cai DH, Li QN, Chen H, Deng WM, He L, Yang L. Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcif Tissue Int. 2010; 86(6):495–501. PMID: 20390406.

20. Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schütz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010; 11(6):517–531. PMID: 20519123.

21. Weinstein RS, O'Brien CA, Almeida M, Zhao H, Roberson PK, Jilka V, Manolagas SC. Osteoprotegerin prevents glucocorticoidinduced osteocyte apoptosis in mice. Endocrinology. 2011; 152(9):3323–3331. PMID: 21771887.

22. Cui L, Li T, Liu Y, Zhou L, Li P, Xu B, Huang L, Chen Y, Liu Y, Tian X, Jee WS, Wu T. Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS One. 2012; 7(4):e34647. PMID: 22493705.

23. Pennisi P, D'Alcamo MA, Leonetti C, Clementi A, Cutuli VM, Riccobene S, Parisi N, Fiore CE. Supplementation of L-arginine prevents glucocorticoid-induced reduction of bone growth and bone turnover abnormalities in a growing rat model. J Bone Miner Metab. 2005; 23(2):134–139. PMID: 15750691.

24. Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, Kostenuik PJ, Erben RG, Hofbauer LC. Inhibition of receptor activator of NF-kappa B ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009; 175(2):473–478. PMID: 19590040.
25. Yoon HY, Won YY, Chung YS. Poncirin prevents bone loss in glucocorticoid-induced osteoporosis in vivo and in vitro. J Bone Miner Metab. 2012; 3(5):509–516. PMID: 22407507.
26. Reddy GK. Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg. 2004; 22(2):141–150. PMID: 15165389.

27. Lewiecki EM, Laster AJ. Clinical applications of vertebral fracture assessment by dual-energy x-ray absorptiometry. J Clin Endocrinol Metab. 2006; 91(11):4215–4222. PMID: 16940447.

28. Green D, Wallace H. Late effects of childhood cancer. 2003. CRC Press.
29. Freidouni M, Nejati H, Salimi M, Bayat M, Amini A, Noruzian M, Asgharie MA, Rezaian M. Evaluating glucocorticoid administration on biomechanical properties of rats' tibial diaphysis. Iran Red Crescent Med J. 2015; 17(3):e19389. PMID: 26019900.

30. Fridoni M, Masteri Farahani R, Nejati H, Salimi M, Gharavi SM, Bayat M, Amini A, Torkman G, Bayat S. Evaluation of the effects of LLLT on biomechanical properties of tibial diaphysis in two rat models of experimental osteoporosis by a three point bending test. Lasers Med Sci. 2015; 30(3):1117–1125. PMID: 25616711.

31. Dadpay M, Sharifian Z, Bayat M, Bayat M, Dabbagh A. Effects of pulsed infra-red low level-laser irradiation on open skin wound healing of healthy and streptozotocin-induced diabetic rats by biomechanical evaluation. J Photochem Photobiol B. 2012; 111:1–8. PMID: 22494918.

32. Bayat M, Abdi S, Javadieh F, Mohsenifar Z, Rashid MR. The effects of low-level laser therapy on bone in diabetic and nondiabetic rats. Photomed Laser Surg. 2009; 27(5):703–708. PMID: 19698018.

33. McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008; 20(2):131–137. PMID: 18349741.

34. Henneicke H, Herrmann M, Kalak R, Brennan-Speranza TC, Heinevetter U, Bertollo N, Day RE, Huscher D, Buttgereit F, Dunstan CR, Seibel MJ, Zhou H. Corticosterone selectively targets endo-cortical surfaces by an osteoblast-dependent mechanism. Bone. 2011; 49(4):733–742. PMID: 21722764.

Figure 1
Schematic representation of the load-deformation curve of L1 vertebral body. The graph illustrates various biomechanical characteristics derived from the material-testing machine and its computer, in which an increasing load is placed on the tissue while isplacement is monitored. Bending stiffness, shown as the maximum slope on the linear portion of the load vs. the displacement curve, is derived from the linear portion of the curve. Energy absorption is the area under the curve.
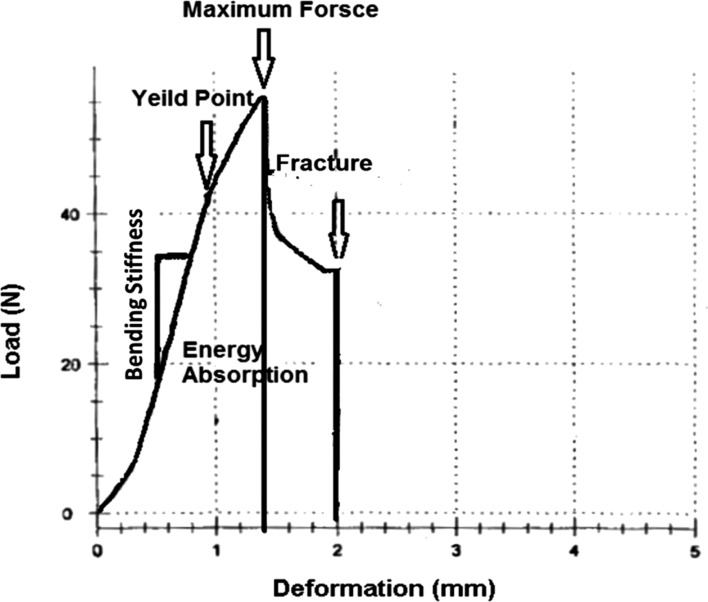
Figure 2
The comparison of the mean values(±SEM ) of the weights of rats at the beginning of the study and at the end of the study; 0.05, 0.01, 0.001.
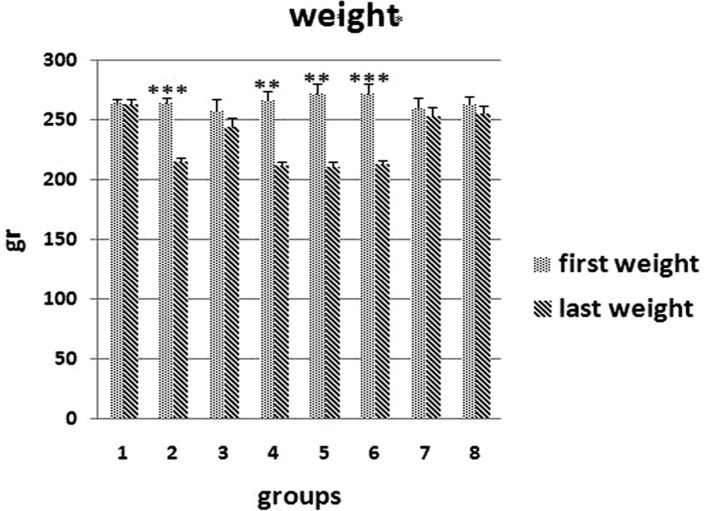
Figure 3
Bending stiffness (N/mm2) of L1 vertebral body of the groups studied. Values are mean±SEM for five animals per group. Statistical comparisons made against control rats (ANOVA test). control group=control (1); 2=Dex 7 mg/kg, I; 3=Dex 0.7 mg/kg I,4=Met 7 mg/kg, I; 5=Met 5 mg/kg, II; 6=Dex 7 mg/kg, III; 7=Dex 0.7 mg/kg, III; 8=low-level laser-treated rats; 0.05, 0.01, 0.001.
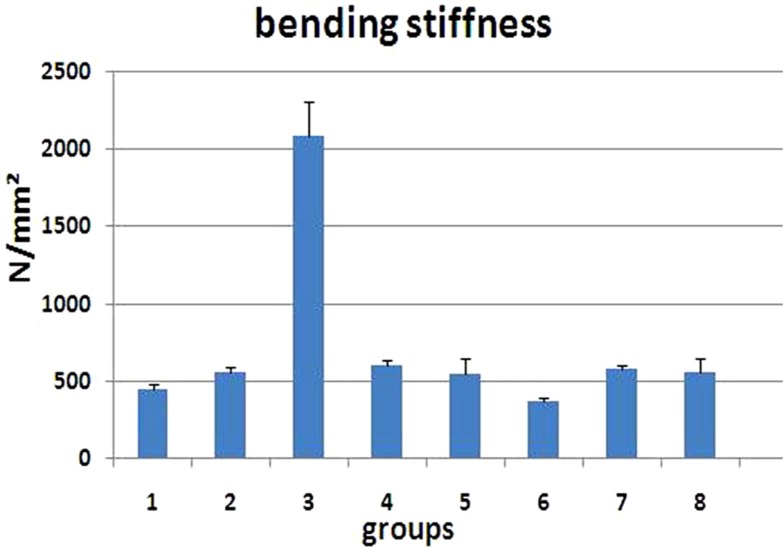
Figure 4
Maximum force (N) of L1 vertebral body of the groups studied. Values are mean±SEM for five animals per group. Statistical comparisons made against control rats (ANOVA test). control group=control (1); 2=Dex 7mg/kg, I; 3=Dex 0.7 mg/kg I, 4=Met 7 mg/kg, I; 5=Met 5 mg/kg, II; 6=Dex 7 mg/kg, III; 7=Dex 0.7 mg/kg, III; 8=low-level laser-treated rats; 0.05, 0.01, 0.001.
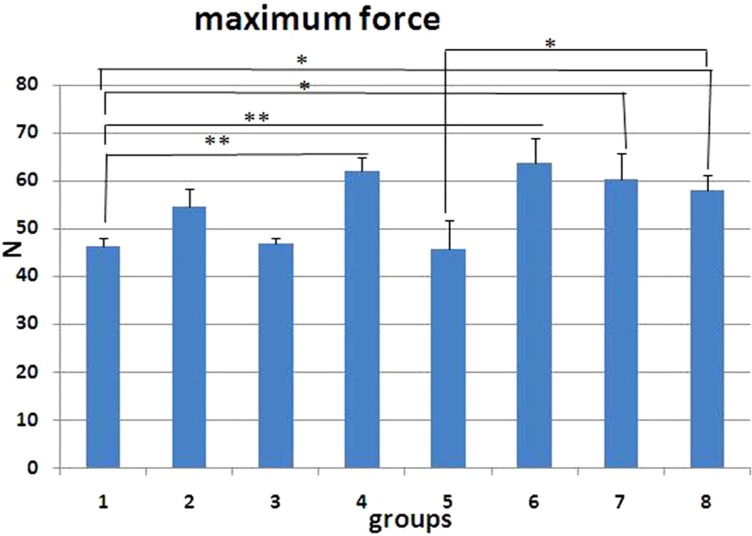
Figure 5
Stress high load (N/mm2) of L1 vertebral body of the groups studied. Values are mean±SEM for five animals per group. Statistical comparisons made against control rats (ANOVA test). control group=control (1); 2=Dex 7 mg/kg, I; 3=Dex 0.7 mg/kg I, 4=Met 7 mg/kg, I; 5=Met 5 mg/kg, II; 6=Dex 7 mg/kg, III; 7=Dex 0.7 mg/kg, III; 8=low-level laser-treated rats; 0.05, 0.01, 0.001.
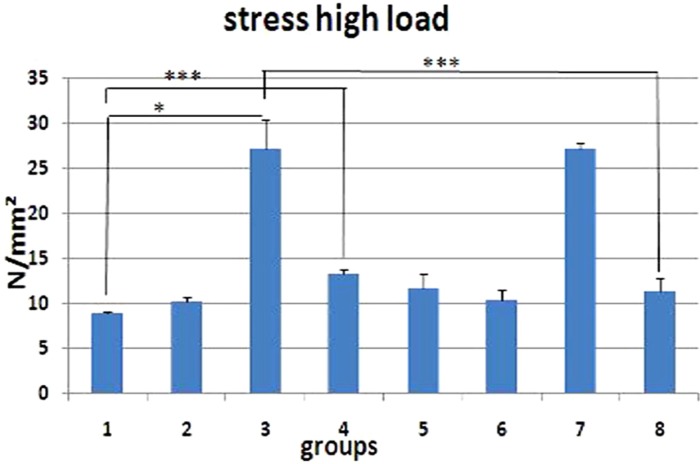
Figure 6
Energy absorption up to maximum force (N mm) of L1 vertebral body of the groups studied. Values are mean±SEM for five animals per group. Statistical comparisons made against control rats (Statistical comparisons made against control rats (Kruscall wallis and Mann Whitney U test). *P<0.007. control group=control (1); 2=Dex 7 mg/kg, I; 3=Dex 0.7 mg/kg I, 4=Met 7 mg/kg, I; 5=Met 5 mg/kg, II; 6=Dex 7 mg/kg, III; 7=Dex 0.7 mg/kg, III; 8=low-level laser-treated rats; 0.05, 0.01, 0.001.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download