Abstract
Procedures involving complex surgical techniques in rats, such as placement of abdominal aortic graft require extended duration of surgical anesthesia, which often can be achieved by repeated administrations of xylazine-ketamine combination. However such repeated anesthetic administration, in addition to being technically challenging, may be associated with potential adverse events due to cumulative effects of anesthesia. We report here the feasibility of using urethane at low dose (~1/10 the recommended anesthetic dose) in combination with a xylazine-ketamine mix to achieve an extended duration of surgical anesthesia in rats. The anesthesia induction phase was quick and smooth with an optimal phase of surgical anesthesia achieved for up to 90 minutes, which was significantly higher compared to that achieved with use of only xylazine-ketamine combination. The rectal temperature, heart rate and respiratory rate were within the physiological range with an uneventful recovery phase. Post surgery the rats were followed up to 3 months without any evidence of tumor or any other adverse effects related to the use of the urethane anesthetic combination. We conclude that low dose urethane can be effectively used in combination with xylazine and ketamine to achieve extended duration of surgical anesthesia up to 90 minutes in rats.
Rodents are extensively used in biomedical research wherein they have proved to be very valuable in addressing early proof of scientific concepts [123456789]. This is specifically true in the area of regenerative medicine, where rodents (specifically rats) are suitable models in pilot screening to test vascular scaffolds developed using regenerative medicine approach [510111213]. However the micro-surgical procedures required to place the vascular scaffolds in the rat abdominal blood vessels are technically challenging and often require extended duration of surgical anesthesia (~70-90 minutes; Unpublished data). Although this can be achieved by inhalation anesthetics, the potential maneuvers required during micro-surgical procedures, the wide area of access to the abdominal cavity leading to pressure on diaphragm and the potential exposure of the surgeon to inhalant anesthetics limit the use of inhalation anesthesia for such procedures [14151617]. Contrary to this the most widely used injectable anesthetics such as xylazine and ketamine combination produce a limited duration of surgical anesthesia (~40 mins) [181920], which is not adequate to complete technically challenging micro-surgical procedures. This necessitates repeated anesthetic administration, which in case of open abdominal surgery will be limited to intravenous administration and often leading to potential anesthetic over dose or adverse events due to cumulative effects of anesthesia [182021]. Hence we explored the possibility of extending the duration of surgical anesthesia achieved by xylazine and ketamine combination.
Urethane is a well-established anesthetic agent, which is extensively used alone or in combination to induce surgical anesthesia in laboratory animals for both acute and chronic procedures [222324252627282930]. The preference towards using urethane for acute non-recovery procedures is due to its ability to produce long lasting surgical plane of anesthesia (often wrongly referred to as irreversible anesthesia) with least effects on factors regulating cardiorespiratory mechanics [1931323334]. Hence in the current study we assessed the feasibility of using urethane in combination with xylazine and ketamine to achieve extended duration of surgical anesthesia to facilitate the micro-surgical procedure of placing vascular scaffolds in the rat abdominal blood vessels.
Urethane (U 2500) was purchased from Sigma-Aldrich. A 0.65 g/mL solution of Urethane was prepared using sterile distilled water. Vetalar (10% Ketamine) and Xylazine (2% Xylazine) were sourced from the designated veterinarian.
Fisher rats bred at rodent facility of University College Cork (originally sourced from Charles River Laboratories, UK) were used in this study. Rats used in experiments were 14-20 weeks of age weighing 250-380 g. The experimental procedures on animals were reviewed and approved by Animal Use Ethics Committee at University College Cork.
Based on preliminary trials we decided to use 1/10th the recommended anesthetic dose of urethane (1.3 g/kg) [3536] in our study. While ketamine (90 mg/kg) and xylazine (10 mg/kg) were used at the recommended dose [18192021]. A combination of ketamine and xylazine was prepared as follows: 0.9 mL 10% ketamine solution +0.5 mL of 2% xylazine solution mixed with 0.6 mL of sterile saline. This ketamine-xylazine mix was optimally vortexed and prepared fresh on the day of procedure.
The volume of urethane (0.65 g/mL) was kept constant at 0.1 mL/rat while the volume of ketamine-xylazine mix was varied to match the body weight. For administration 0.1 mL of urethane was drawn first into a 1 mL syringe, and the ketamine-xylazine mix was then drawn into the syringe to the required pre-calculated volume. Following this the entire mixture was administered to the rat by the intraperitoneal route.
The duration of anesthetic induction, surgical plane and recovery time were recorded. The rats were continuously observed throughout the procedure and all observations were recoded. Additionally the body temperature, heart rate and respiratory rate were also continuously monitored and recorded. These parameters were compared with rats anesthetized using combination of ketamine (90 mg/kg) and xylazine (10 mg/kg) without urethane for Balloon injury of Carotid artery procedure.
The rats were observed up to 1-3 months post procedure following which detailed post mortem examination was performed to evaluate any anatomical adverse effects of urethane use.
Data from 57 rats used for abdominal aortic graft or SHAM procedures and 24 rats used for Balloon injury of Carotid artery procedure were analysed. The data are presented as Mean±SD and were compared by un-paired t-test using Graph Pad Prism Version 5 (Graph-Pad Software Inc, San Diego, USA). Statistical significance threshold was set at P≤0.05.
Use of urethane-ketamine-xylazine (UKX) combination resulted in smooth induction of anesthesia similar to that achieved with use of ketamine-xylazine (KX) combination. The time for induction of anesthesia in UKX group (4.25±0.87 minutes) was significantly (P<0.0001) lesser compared to that required with use of KX combination (Figure 1A). While the duration of surgical plane of anesthesia and recovery from anesthesia observed using UKX was significantly (P<0.0001) longer compared to KX (Figure 1B,C). The duration of surgical anesthesia achieved by UKX combination ranged from 55 to 90 minutes with a mean duration of 73.93±9 minutes, which was almost 2 folds higher duration of surgical anesthesia compared to that achieved by KX combination (38.21±4.61 minutes) (Figure 1B). The longer duration of surgical anesthesia concurrently resulted in a prolonged recovery period from anesthesia in rats administered UKX combination, which ranged from 180-280 minutes, with a mean recovery duration of 227.46±32.6 minutes, which was 2.2 fold longer than that observed with the KX combination (Figure 1C).
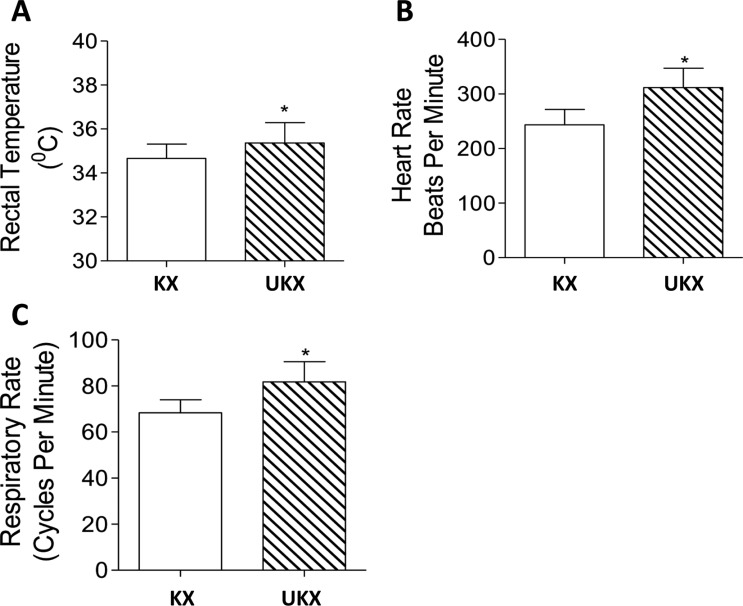
Physiological parameters, such as rectal temperature (Figure 2A), heart rate (Figure 2B) and respiratory rate (Figure 2B) were within the normal range in either group. However the physiological parameters measured were statistically better in UKX group compared to KX group (Figure 2). The recovery phase of anesthesia was smooth and uneventful in both the groups. The rats were followed up to 3 months post anesthesia administration and culled. Detailed postmortem examination did not reveal any gross abnormalities, indicating that urethane use in rats at the indicated dose is feasible and safe.
We evaluated the potential of low dose urethane in combination with ketamine and xylazine to achieve extended duration of surgical anesthesia in rats. Urethane is often used as an anesthetic for acute non-recovery procedures in rodents, specifically for procedures involving surgical intervention on cardiovascular system [3536]. The preference of urethane is due to its minimal adverse effects on cardiovascular and respiratory physiology in addition to its superior analgesic effects [27373839]. However use of urethane for chronic recovery procedures has been limited due its carcinogenic and mutagenic effects evident on repeated use [404142434445]. However whether such carcinogenic and mutagenic effects can be observed on single or sub-anesthetic dose administration of anesthetic urethane (ethyl urethane) is not documented.
A hugely positive feature of urethane anesthesia in laboratory animals is its ability to produce prolonged duration of surgical anesthesia, [1931323334] which is often vaguely termed as irreversible anesthesia. Considering these facts on urethane, in the present study we hypothesized that use of urethane in combination with ketamine and xylazine (which is the most widely used anesthetic approach in laboratory rodents) can help to achieve extended duration of surgical anesthesia. Based on a pilot study we limited the urethane dose to 1/10th of its recommended anesthetic dose (1.3 g/kg) in rodents [3536]. It was feasible to achieve up to 90 minutes of surgical anesthesia using our combination of urethane, ketamine and xylazine (UKX), which to best of our knowledge is not previously reported. The smooth and rapid induction of anesthesia with UKX with minimal effects on physiological parameters is a highly desirable anesthetic feature and we recommend this approach for surgical procedures in rats requiring 50-90 minutes of surgical anesthesia. Which in our opinion is favorable and preferred over repeated administration of ketaminexylazine combination. Although we did observe prolonged duration of recovery from anesthesia using UKX, which may not be desirable, however the fact that the recovery phase was smooth and without any adverse events is indeed promising for the wider use of the UKX anesthetic combination. However in surgical procedures involving major vascular structures in the abdomen, a prolonged recover period post surgery may be beneficial as this helps achieve optimal stabilization of the vascular anastomosis site. Further at 3 months of follow up we did not observe any evidence of tumors or any other adverse events, supports our claim on utility of UKX combination to induce anesthesia for prolonged surgical recovery procedures in rats. Hence use of urethane at sub-anesthetic dose in combination with xylazine and ketamine is safe. Moreover the superior analgesic effects of urethane may be additionally beneficial during and post complex surgical procedures. Although we did not evaluate a dose dependent effects of urethane, as our required duration of surgical anesthesia was met with this combination, nevertheless it will be interesting to assess in further studies, if altering the relative ratio of urethane in UKX mix will facilitate further extension of surgical plane of anesthesia. Moreover such an approach may also help to reduce the dose of ketamine-xylazine, which in our opinion will be beneficial during surgical procedures on cardiovascular and/or respiratory system in rodents.
Acknowledgment
We acknowledge the support from Irish Blood Transfusion Service (AJPC), University College Dublin-Seed funding (AHSK) and Stemcology (AHSK).
References
1. Arun KH, Kaul CL, Ramarao P. AT1 receptors and L-type calcium channels: functional coupling in supersensitivity to angiotensin II in diabetic rats. Cardiovasc Res. 2005; 65(2):374–386. PMID: 15639476.
2. Arun KH, Kaul CL, Poduri R. Tempol augments angiotensin IIinduced AT2 receptor-mediated relaxation in diabetic rat thoracic aorta. J Hypertens. 2004; 22(11):2143–2152. PMID: 15480099.

3. Piche M, Watanabe N, Hotta H. Regulation of gastric motility and blood flow during acute nociceptive stimulation of the paraspinal muscles in urethane-anaesthetised rats. J Physiol Sci. 2014; 64(1):37–46. PMID: 24037728.

4. Habara H, Hayashi Y, Inomata N, Niijima A, Kangawa K. Organspecific activation of the gastric branch of the efferent vagus nerve by ghrelin in urethane-anesthetized rats. J Pharmacol Sci. 2014; 124(1):31–39. PMID: 24366191.

5. Tran RT, Choy WM, Cao H, Qattan I, Chiao JC, Ip WY, Yeung KW, Yang J. Fabrication and characterization of biomimetic multichanneled crosslinked-urethane-doped polyester tissue engineered nerve guides. J Biomed Mater Res A. 2014; 102(8):2793–2804. PMID: 24115502.

6. Krynauw H, Bruchmuller L, Bezuidenhout D, Zilla P, Franz T. Degradation-induced changes of mechanical properties of an electro-spun polyester-urethane scaffold for soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2011; 99(2):359–368. PMID: 21948379.

7. Chiono V, Sartori S, Rechichi A, Tonda-Turo C, Vozzi G, Vozzi F, D'Acunto M, Salvadori C, Dini F, Barsotti G, Carlucci F, Burchielli S, Nicolino S, Audisio C, Perroteau I, Giusti P, Ciardelli G. Poly(ester urethane) guides for peripheral nerve regeneration. Macromol Biosci. 2011; 11(2):245–256. PMID: 21104881.

8. Kumar AH, Martin K, Turner EC, Buneker CK, Dorgham K, Deterre P, Caplice NM. Role of CX3CR1 receptor in monocyte/macrophage driven neovascularization. PLoS One. 2013; 8(2):e57230. PMID: 23437346.

9. Clover AJ, Kumar AH, Caplice NM. Deficiency of CX3CR1 delays burn wound healing and is associated with reduced myeloid cell recruitment and decreased sub-dermal angiogenesis. Burns. 2011; 37(8):1386–1393. PMID: 21924836.

10. Shen Z, Chen J, Kang C, Gong C, Zhu Y. Engineered hypopharynx from coculture of epithelial cells and fibroblasts using poly(ester urethane) as substratum. Biomed Res Int. 2013; 2013:138504. PMID: 24455669.

11. Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR. Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials. 2010; 31(15):4249–4258. PMID: 20188411.

12. Boissard CI, Bourban PE, Tami AE, Alini M, Eglin D. Nanohydroxyapatite/poly(ester urethane) scaffold for bone tissue engineering. Acta Biomater. 2009; 5(9):3316–3327. PMID: 19442765.

13. Field JR, Gunatillake P, Adhikari R, Ramshaw JA, Werkmeister JA. Use of biodegradable urethane-based adhesives to appose meniscal defect edges in an ovine model: a preliminary study. Aust Vet J. 2008; 86(6):229–234. PMID: 18498559.

14. Furtado KS, Andrade FO. Comparison of the beneficial and adverse effects of inhaled and injectable anaesthetics: A minireview. OA Anaesthetics. 2013; 1(2):20.

15. Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, Hanig JP, Paule MG, Slikker W Jr, Wang C. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol. 2011; 33(5):592–597. PMID: 21708249.

16. Agostoni M, Fanti L, Gemma M, Pasculli N, Beretta L, Testoni PA. Adverse events during monitored anesthesia care for GI endoscopy: an 8-year experience. Gastrointest Endosc. 2011; 74(2):266–275. PMID: 21704990.

17. Sneyd JR, Holmes KA. Inhalational or total intravenous anaesthesia: is total intravenous anaesthesia useful and are there economic benefits? Curr Opin Anaesthesiol. 2011; 24(2):182–187. PMID: 21252648.

18. Ritschl LM, Fichter AM, Haberle S, von Bomhard A, Mitchell DA, Wolff KD, Mucke T. Ketamine-Xylazine Anesthesia in Rats: Intraperitoneal versus Intravenous Administration Using a Microsurgical Femoral Vein Access. J Reconstr Microsurg. 2015; 31(5):343–347. PMID: 25702886.

19. Giroux MC, Helie P, Burns P, Vachon P. Anesthetic and pathological changes following high doses of ketamine and xylazine in Sprague Dawley rats. Exp Anim. 2015; 64(3):253–260. PMID: 25818316.

20. Albrecht M, Henke J, Tacke S, Markert M, Guth B. Effects of isoflurane, ketamine-xylazine and a combination of medetomidine, midazolam and fentanyl on physiological variables continuously measured by telemetry in Wistar rats. BMC Vet Res. 2015; 10:198. PMID: 25149627.

21. Dittmar MS, Fehm NP, Vatankhah B, Horn M. Ketamine/xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp Med. 2004; 54(6):652–655. PMID: 15679263.
22. Wanger T, Takagaki K, Lippert MT, Goldschmidt J, Ohl FW. Wave propagation of cortical population activity under urethane anesthesia is state dependent. BMC Neurosci. 2013; 14:78. PMID: 23902414.

23. Wang K, Zhu H, Chen CY, Li P, Jin CH, Wang ZL, Jiang S, Hua TM. [Effects of ketamine and urethane on stimulation-induced cfos expression in neurons of cat visual cortex]. Dongwuxue Yanjiu. 2013; 34(6):582–588. PMID: 24415690.
24. Aizawa N, Ogawa S, Sugiyama R, Homma Y, Igawa Y. Influence of urethane-anesthesia on the effect of resiniferatoxin treatment on bladder function in rats with spinal cord injury. Neurourol Urodyn. 2015; 34(3):274–279. PMID: 24375785.

25. Liu X, Li R, Yang Z, Hudetz AG, Li SJ. Differential effect of isoflurane, medetomidine, and urethane on BOLD responses to acute levo-tetrahydropalmatine in the rat. Magn Reson Med. 2012; 68(2):552–559. PMID: 22213080.

26. Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn. 2010; 29(7):1344–1349. PMID: 20127833.

27. Sharma AV, Wolansky T, Dickson CT. A comparison of sleeplike slow oscillations in the hippocampus under ketamine and urethane anesthesia. J Neurophysiol. 2010; 104(2):932–939. PMID: 20538775.

28. Bertera FM, Di Verniero CA, Mayer MA, Bramuglia GF, Taira CA, Höcht C. Is urethane-chloralose anaesthesia appropriate for pharmacokinetic-pharmacodynamic assessment? Studies with carvedilol. J Pharmacol Toxicol Methods. 2009; 59(1):13–20. PMID: 18973819.

29. Princi T, Delbello G, Grill V. Experimental urethane anaesthesia prevents digoxin intoxication: electrocardiographic and histological study in rabbit. Pharmacol Res. 2000; 42(4):355–359. PMID: 10987996.

30. el-Mas MM, Abdel-Rahman AA. Contrasting effects of urethane, ketamine, and thiopental anesthesia on ethanol-clonidine hemodynamic interaction. Alcohol Clin Exp Res. 1997; 21(1):19–27. PMID: 9046368.

31. Gonsenhauser I, Wilson CG, Han F, Strohl KP, Dick TE. Strain differences in murine ventilatory behavior persist after urethane anesthesia. J Appl Physiol. 1985; 97(3):888–894. PMID: 15333626.

32. El Desoky ES, Abdulla MM. Studying long-term effect of phenytoin either alone or combined with ascorbic acid on the anesthetic effect of urethane in rats. Fundam Clin Pharmacol. 2004; 18(2):153–156. PMID: 15066128.

33. Zorniak M, Mitrega K, Bialka S, Porc M, Krzeminski TF. Comparison of thiopental, urethane, and pentobarbital in the study of experimental cardiology in rats in vivo. J Cardiovasc Pharmacol. 2010; 56(1):38–44. PMID: 20351562.
34. Clement EA, Richard A, Thwaites M, Ailon J, Peters S, Dickson CT. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS One. 2008; 3(4):e2004. PMID: 18414674.

35. Devonshire IM, Grandy TH, Dommett EJ, Greenfield SA. Effects of urethane anaesthesia on sensory processing in the rat barrel cortex revealed by combined optical imaging and electrophysiology. Eur J Neurosci. 2010; 32(5):786–797. PMID: 20646050.

36. Bauquier SH, Golder FJ. The effects of urethane on the isoflurane minimum alveolar concentration in rats. Lab Anim. 2010; 44(4):323–328. PMID: 20713428.

37. Blatt LK, Lashinger ES, Laping NJ, Su X. Evaluation of pressor and visceromotor reflex responses to bladder distension in urethane anesthetized rats. Neurourol Urodyn. 2009; 28(5):442–446. PMID: 19030181.

38. Albrecht D, Blühdorn R, Siegmund H, Berger H, Calo' G. Inhibitory action of nociceptin/orphanin FQ on functionally different thalamic neurons in urethane-anaesthetized rats. Br J Pharmacol. 2001; 134(2):333–342. PMID: 11564651.

39. Soehring K, Frahm M. [Pharmacology of alkylpolyethylene oxide derivatives. VII. Effect on circulation and respiration in cats under urethane and evipan sodium narcosis in comparison with other local analgesics]. Arzneimittelforschung. 1955; 5(11):655–662. PMID: 13293062.
40. Duenas-García IE, Santos-Cruz LF, Castaneda-Partida L, Castaneda-Sortibran AN, Ordaz-Tellez MG, Sanchez-Santos A, Duran-Díaz A, Rodríguez-Arnaiz R, Heres-Pulido ME. Interactions of sulforaphane and dimethyl sulfoxide with methyl methanesulfonate, urethane, 4-nitroquinoline-1-oxide and hydrogen peroxide in the Drosophila melanogaster wing spot test. Food Chem Toxicol. 2012; 50(12):4479–4486. PMID: 23026699.
41. Beland FA, Benson RW, Mellick PW, Kovatch RM, Roberts DW, Fang JL, Doerge DR. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem Toxicol. 2005; 43(1):1–19. PMID: 15582191.

42. Sakano K, Oikawa S, Hiraku Y, Kawanishi S. Metabolism of carcinogenic urethane to nitric oxide is involved in oxidative DNA damage. Free Radic Biol Med. 2002; 33(5):703–714. PMID: 12208357.

43. Chan PC. NTP technical report on toxicity studies of urethane in drinking water and urethane in 5% ethanol administered to F344/N rats and B6C3F1 mice. Toxic Rep Ser. 1996; 52:1–91. PMID: 11803705.
44. Vorobtsova IE, Kitaev EM. Urethane-induced lung adenomas in the first-generation progeny of irradiated male mice. Carcinogenesis. 1988; 9(11):1931–1934. PMID: 3180332.

45. Ward JM, Rehm S, Devor D, Hennings H, Wenk ML. Differential carcinogenic effects of intraperitoneal initiation with 7,12-dimethylbenz(a)anthracene or urethane and topical promotion with 12-O-tetradecanoylphorbol-13-acetate in skin and internal tissues of female SENCAR and BALB/c mice. Environ Health Perspect. 1986; 68:61–68. PMID: 3096710.

Figure 1
The bar graphs represent the anesthetic Induction time (A), duration of surgical plane of anesthesia (B) and duration of recovery from anesthesia (C) in fisher rats administered a combination of ketamine-xylazine (KX) or Urethane-ketamine-xylazine (UKX) by intraperitoneal route. Data is presented as Mean±SD. *P<0.0001 vs KX group.
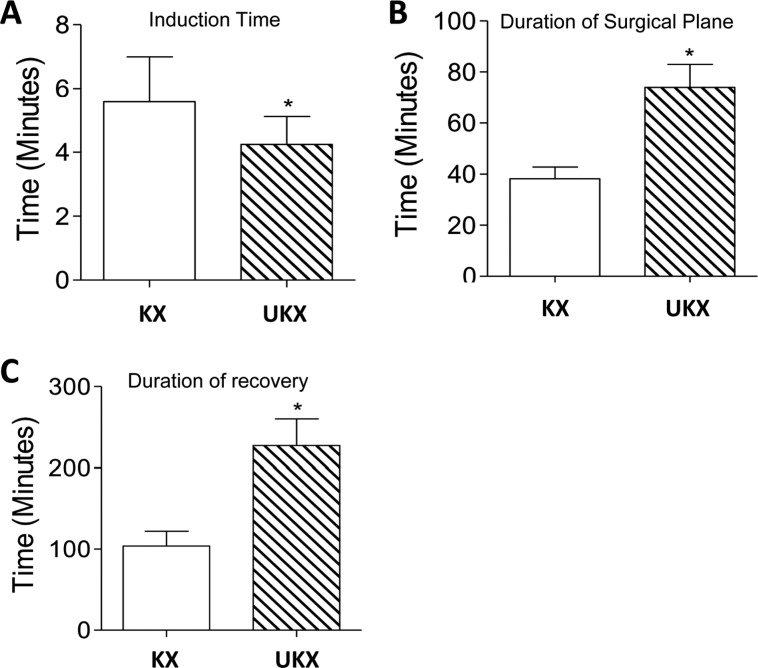
Figure 2
The bar graphs represent the rectal temperature (A), heart rate (B) and respiratory rate (C) in fisher rats administered a combination of ketamine-xylazine (KX) or Urethane-ketamine-xylazine (UKX) by intraperitoneal route. Data is presented as Mean±SD. *P<0.01 vs KX group (2A), P<0.0001 vs KX group (2B,2C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download