Abstract
The common marmoset (Callithrix jacchus) is a small-bodied, popular New World monkey and is used widely in reproductive biology, neuroscience, and drug development, due to its comparative ease of handling, high reproductive efficiency, and its unique behavioral characters. In this review, we discuss the marmoset models in Parkinson's disease (PD), which is a neurological movement disorder primarily resulting from a degeneration of dopaminergic neurons with clinical features of tremor, rigidity, postural instability, and akinesia. The most common PD models involve the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or 6-hydroxydopamine to study the pathogenesis and to evaluate novel therapies. Following the systemic or local administration of these neurotoxins, the marmosets with very severe Parkinson's symptoms are recommended to be placed in an intensive care unit with artificial feeding to increase survival rate. All procedures with MPTP should be conducted in a special room with enclosed cages under negative-pressure by trained researchers with personal protection. Behavioral tests are conducted to provide an external measure of the brain pathology. Along with several biomarkers, including α-synuclein and DJ-1, non-invasive neuroimaging techniques such as positron emission tomography and magnetic resonance imaging are used to evaluate the functional changes associated with PD. With the recent growing interest in potential and novel therapies such as stem cell and gene therapy for PD in Korea, the marmoset can be considered as a suitable non-human primate model in PD research to bridge the gap between rodent studies and clinical applications.
Parkinson's disease (PD) is one of the most common neurodegenerative movement disorder, and affects ~2% of the world's population aged over 65 [1]. It is characterized by insufficient production of dopamine (DA) from the substantia nigra (SN) area of the brain, leading to asymmetric onset of bradykinesia, resting tremor, rigidity and postural instability. Pathologically, the hallmarks of PD are evolutional nigrostriatal DA neurodegeneration in the brain and the presence of cytosolic filamentous inclusions known as Lewy bodies (LBs) and Lewy neurites in surviving nigral DA cells [234]. Recent findings indicate that the mechanisms of DA neuron degeneration and death have been linked to mitochondrial dysfunction, oxidative stress, inflammation, and apoptosis [25]. In addition, PD also affects many other brain regions, such as the dorsal motor nucleus of the vagus, the nucleus basalis of Meynert, and the locus coeruleus [67].
PD is a result of complex interactions between environmental and genetic factors associated with a pathogenic mechanism and thus cannot be well studied using simple in vitro models [8]. For the past several decades, animal models of PD with their own strengths and weaknesses have been widely used to investigate the pathogenesis as well as possible innovative therapeutic approaches for this neurodegenerative disorder [9]. Experimental models of PD should reflect pathological, biochemical, and clinical features of PD including the lesions in both DA and non-DA systems. Although none of the current models show all the features of PD, animal models have contributed significantly to our current understanding of the pathological mechanisms of the disease and to the development of new therapeutic strategies in PD [10]. In this review, we examine the use of the common marmoset (Callithrix jacchus) (Figure 1A) treated with systemic or local administration of neurotoxins as an animal model to bridge the gap between rodent studies and clinical applications for the purpose of studying the pathogenesis and evaluating novel therapies for PD.
The marmoset is a small New World primate that is originally from the Amazon basin of Brazil [11]. It is a relatively small animal, with an average height of 20-30 cm and weigh of 200-600 g (10-15-fold less than the 5-10 kg macaque). According to the United States National Research Council's "Guide for the Care and Use of Laboratory Animals", a small cage (Figure 1B) with a minimum height of 76.2 cm and a minimum floor area of 0.20 m2 is recommended for a breeding pair of marmosets. According to the European guidelines, marmosets should be kept in controlled facilities (23-28℃, 45-70% humidity, and 12 h light/dark cycle) since the animals originate from tropical rain forests [12].
The marmosets eat fresh fruit, bread, eggs, and nuts, and have high protein intake. Along with biscuits and condensed milk as remuneration food, a commercial diet must be supplemented with vitamin D3 since they require a large amount of vitamin D3 (e.g. "New World Primate Diet" by Harlan Teklad) [12]. The marmosets have high reproductive efficiency, with similarities to the human ovarian cycle (approximately 28 days), an early onset of puberty (around 1.5 years old), a relatively short gestation period (145-148 days), a relatively large litter sizes (2-3 offspring per delivery), and a relatively large number of deliveries (twice a year) [11]. They live in stable families of 1-2 breeding females, a breeding male, their offspring, and their adult relatives [13]. For toxicokinetic or clinical pathology studies, blood samples are usually collected via the femoral vein and occasionally the tail vein. Less than 15% of the circulating blood volume is recommended for blood collection within one month for the marmoset [12].
Because of its small size, ease of handling, and unique biological characteristics [14], the marmoset has become an important primate model in various areas of biomedical research such as neuroscience, toxicology, reproductive biology and regenerative medicine [15]. Importantly, the use of marmosets can lead to significant reductions in material requirements due to its small size [12].
Transient parkinsonian-like states have been generated in various animal species from drosophila [16], to mice [17], rats [18], cats [19], minipigs [20], sheep [21], New World [222324], and Old World monkeys [4]. For many researchers, the mouse is a popular choice for behavioral assessments and screening for the effects of drug treatments due to a lack of resources and trained personnel for the monkey model. Monkeys have many similarities to humans in terms of developmental processes, brain anatomy/function, and social behaviors, hence, research on monkeys play an important role in the preclinical development process between rodent studies and controlled clinical trials [252627]. In particular, the use of the marmoset monkey requires less ethical justification than the larger "Old World monkeys" [12]. For this reason, there has been increasing interest in the marmoset monkey as a popular monkey species for the development of novel treatments for PD such as neurotrophic factors [28], DA reuptake inhibitors [29], and neurotransplantation [30].
Current animal models of PD include genetic and neurotoxic models. The genetic models are created primarily based on genes identified in potential mechanisms involved in the onset and propagation of PD in humans [931]. Over-expression of proteins such as á-synuclein and DJ-1 using viral vectors results in great practical significance of PD symptoms, leading to preclinical evaluations of various therapies for PD [932]. Recently, several genetically modified non-human primate (NHP) models were developed through the introduction of exogenous genes into NHP genomes or the alteration of endogenous NHP genes [3334]. This progress in knowledge and technology enable the production of transgenic marmoset models with clear PD phenotypes, which will have great practical significance for understanding PD pathophysiology. However, studies on the pathogenesis of the marmoset PD models can take a long time due to the long lifespans of the marmosets compared with rodents. Currently available genetic models do not completely induce appreciable neurodegeneration and PD phenotypes [35], whereas the neurotoxic models are used to damage the nigrostriatal pathway [10]. The marmoset model is a recognized model of PD using neurotoxins that induce the selective degeneration of nigrostriatal neurons [223637]. The most commonly used toxins are 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA), which reproduce the pathological and behavioral changes of the human disease in rodents or NHPs. These models can be developed by the systemic or local administration of neurotoxins depending on the type of agent used and the species involved [9].
MPTP was found to be a DA neurotoxin in the early 1980s when Langston et al. [38] described the occurrence of severe symptoms similar to PD in several young Californian intravenous drug users following the injection of an analogue of the narcotic meperidine that contained MPTP [39]. The identification of a specific neurotoxin, MPTP, that induces neural damage and other signs of PD in humans [39] has led to the development of valuable mammalian models, including sheep, dogs, guinea pigs, cats, mice, rats, and monkeys, for research on the pathophysiology, etiology, and pathogenesis of PD [4041]. The MPTP-primate model using baboons [42], macaques [4], velvet monkeys [43], squirrel monkeys [44] and marmosets [222324] remains the best model for preclinical evaluation of the efficacy of anti-parkinsonian therapy although most studies on PD research have been performed in mice due to a lack of resources and trained personnel for monkey models [1045].
Since MPTP is a highly lipophilic compound, it can rapidly cross the blood-brain barrier (BBB). The mechanism underlying MPTP toxicity is the conversion of the MPTP by monoamine oxidase B into the final active toxin cation, 1-methyl-4-phenylpyridinium ion (MPP+), which can enter DA neurons in the substantia nigra pars compacta (SNpc) through the dopamine transporter (DAT). MPP+ induces neurotoxicity primarily by blocking mitochondrial complex I activity, leading to ATP depletion and increased oxidative stress [4647]. In general, a pathological limitation of the mouse MPTP model is the lack of LBs, which is the neuropathological landmark of PD [10], although a few reports have investigated the expression of a LB major constituent (á-synuclein) by modifying the MPTP treatment regimens [9]. Forno et al. [48] also described intraneuronal inclusions reminiscent of LBs in MPTP-injected monkeys. Several MPTP dosing regimens (Table 1) have been used in many studies [222324294950515253].
In comparison to other available PD models, the MPTP model does not require skilled stereotaxic surgery and is known to have the greatest similarity with human PD symptoms biochemically, anatomically and behaviorally [49]. However, in comparison to the 6-OHDA model, the MPTP model shows a dose-dependent risk of mortality from cardiotoxicity within 24 h of the first dose [54]. In addition, strict safety procedures are required with appropriate laboratory safety equipment since systemic MPTP treatment is a severe safety hazard to the personnel handling the animals [41]. Although MPTP itself is not directly harmful, its metabolite MPP+ is tremendously toxic. Since MPTP metabolites are excreted up to 3 days post administration [55], researchers should wear personal protection during all procedures involving MPTP including the preparation, injection, and 3-5 days post injection. Furthermore, all procedures involving MPTP should be conducted in a special room with a fume hood and enclosed cages under negative-pressure (Figure 1C) due to the MPTP aerosols generated from bedding, excreta and animal fur [41].
A catecholamine neurotoxin 6-OHDA is a hydroxylated analogue of DA with high affinity for DAT, which transports the toxin into DA neurons [9], and it can cause selective degeneration of sympathetic adrenergic nerve terminals [56]. The mechanism of 6-OHDA neurotoxicity has often been involved in the production of the reactive oxygen species, H2O2, resulting from the autoxidation of 6-OHDA [9]. It has also been reported that 6-OHDA can accumulate in the mitochondria, where it inhibits the mitochondrial respiratory enzymes (chain complex I), resulting in a damaging depletion of intracellular ATP and consequently cell death [957]. 6-OHDA has been used in rats, cats, guinea pigs, dogs, and monkeys [3140]. Since 6-OHDA does not cross the BBB, direct injection into the region of interest in the brain is required to target specific neurons, with several dosing regimens and different injection sites (Table 2) [5859606162]. Despite the difficulty in targeting small brain structures, the SN is usually targeted for 6-OHDA injection to create a more selective animal model of PD [60]. 6-OHDA lesions can also be made by targeting the striatum or the medial forebrain bundle (MFB). 6-OHDA injection into the striatum induces slow, progressive and partial damage of SNpc neurons over a period of up to 3 weeks, which is less marked than the effects of intra-MFB injection. In contrast, when injected into the MFB, which conveys the efferent fibers from nigral cell bodies to the striatum, 6-OHDA produces a rapid and massive degeneration in the nigrostriatal pathway. When injected into the nigra, a significant loss of striatal DA terminals is established within 2-3 days post-injection [910].
The administration of 6-OHDA is more complicated and time-consuming due to the necessity of stereotaxic surgery (Figure 2) and the difficulty in targeting small brain structures such as the SN or MFB. Additionally, 6-OHDA-treated animals fail to develop LBs, which are eosinophilic inclusions that contain ubiquitinated proteins such as α-synuclein [63]. Unilaterally lesioned animals have been used more often because bilateral injections can induce a far more severe phenotype with marked adipsia, aphagia, and high mortality. The major advantage of the unilateral 6-OHDA model is its feasibility for assessing a variety of different behaviors such as fine motor skills, sensorimotor neglect and body asymmetries. Also, the unilateral injection of 6-OHDA into one hemisphere in the animals leaves the unlesioned side as an internal control, hence, fewer animals are needed in experiments to analyze behavioral deficits [9]. Along with less postoperative care for animals, local 6-OHDA treatment can minimize the risk of inadvertent toxic exposure for researchers associated with systemic MPTP treatments [496465].
Rotenone is a flavonoid found in several plants and is a broad-spectrum pesticide used to kill insects. Like MPTP, it is highly lipophilic, so it readily crosses the BBB and diffuses into cells. Mitochondrial complex I inhibition, selective nigrostriatal neurodegeneration, and α-synuclein-positive cytoplasmic inclusions, which have been reported as key pathological features of clinical PD, were demonstrated in the rotenone model. Therefore, there has been interest in the PD model using rotenone [1066]. However, Betarbet et al. [66] indicated that rotenone displays systemic toxicity and subsequent high mortality rates (~30% of animals) regardless of the administration route. The main limitations of the rotenone model are variability in terms of the percentage of animals that develop a nigrostriatal DA lesion, the extent of the lesion, and the lesion distribution within the striatum [67]. Some animals can be resistant to rotenone (~50% of treated animals display neurodegeneration). This low success rate for the animal model of rotenone makes it necessary to use a larger number of animals at the start of any study. Nonetheless, the PD model involving systemic intravenous delivery of rotenone using osmotic pumps replicates many aspects of the pathology of human PD. Also, chronic intraperitoneal injection of rotenone causes locomotor impairment and neurobehavioral abnormalities characteristic of PD [6869]. Rotenone has been shown to induce DA neurodegeneration and Parkinson-like behavior through many pathogenic pathways including massive reactive oxygen species formation, proteosome activity inhibition, proteolytic stress, α-synuclein phosphorylation and aggregation and Lewy pathology [970]. Therefore, rotenone model can be used for the marmosets along with other classical PD models, MPTP and 6-OHDA.
In order to identify the effects of parkinsonian agents or anti-parkinsonian treatments, body weight, complete blood count, serum biochemistry, gross and microscopic examination of tissues can be measured [71]. Following the induction of Parkinsonian symptoms, there is a critical need for daily management of the marmosets due to potential health threats (adipsia, aphagia, loss of body weight). Usually, the marmosets are allowed to have access to specially formulated pellets, fresh fruits, vegetables and water ad libitum. However, in order to maintain the body weight, welfare and survival of the animals displayed a stable level of akinesia following neurotoxin administration, artificial feedings consisting of a highly nutritious solution made from egg white, sucrose, infant formula, banana, multivitamins, and powdered marmoset pellets should be introduced on a daily basis by trained staffs until the marmosets are able to feed themselves. Also, the marmosets with very severe Parkinson's symptoms can be placed in an intensive care unit with tightly controlled temperature, humidity, and oxygen concentration (Figure 1D) until the marmosets are capable of maintaining food and water intake on their own. However, euthanasia is recommended for animals showing significant body weight loss (>20%) and anorexia.
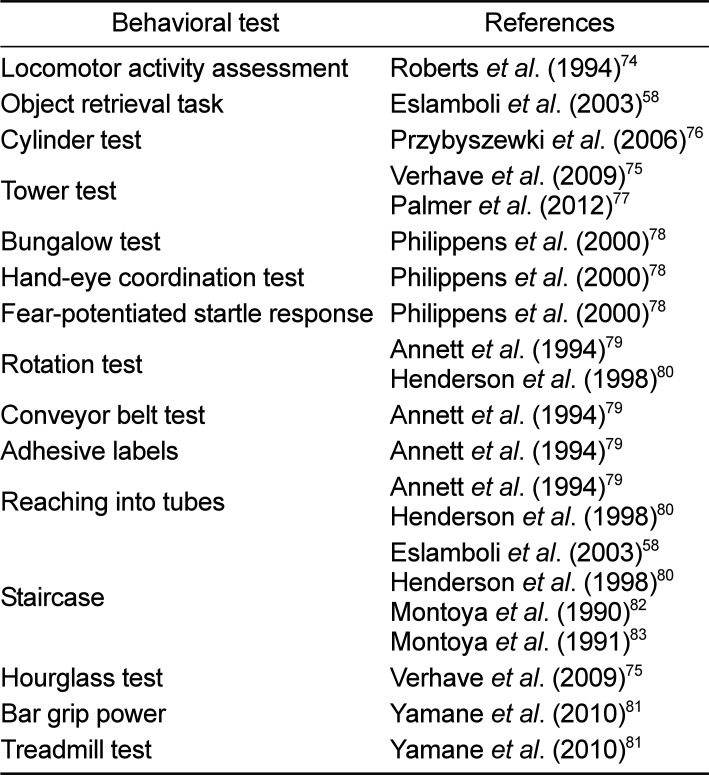
In order to cure Parkinsonian symptoms, highly reproducible animal models of PD should be developed to address all PD-related questions including pathological changes in the brain. First, the occurrence of typical PD symptoms can be recorded using a clinical rating scale (apathy, immobility, muscle rigidity, tremors and inadequate grooming) and for the purpose of scoring abnormal involuntary movements (facial behaviors, full body behaviors, and general severity of involuntary movements and incapacitation due to these movements) (Table 3) [36587273]. These clinical measurements can be performed in a double-blind manner by watching post hoc video-recordings of the animals accompanied by recording spontaneous locomotor activity in the lesioned animals [74]. Behavioral tests provide an external measure of PD-like pathology when assessing PD lesions in animals [71]. Evaluation of Parkinsonian features in marmosets can be conducted by a variety of behavioral tests ranging from simple food-retrieval task to a comprehensive neuropsychological assessment, including cylinder test, tower test (Figure 3), bungalow test, hand-eye coordination test, fear-potentiated startle response, rotation test, conveyor belt test, adhesive labels, reaching into tubes, staircase, hourglass test, bar grip power, and treadmill test (Table 4) [58757677787980818283].
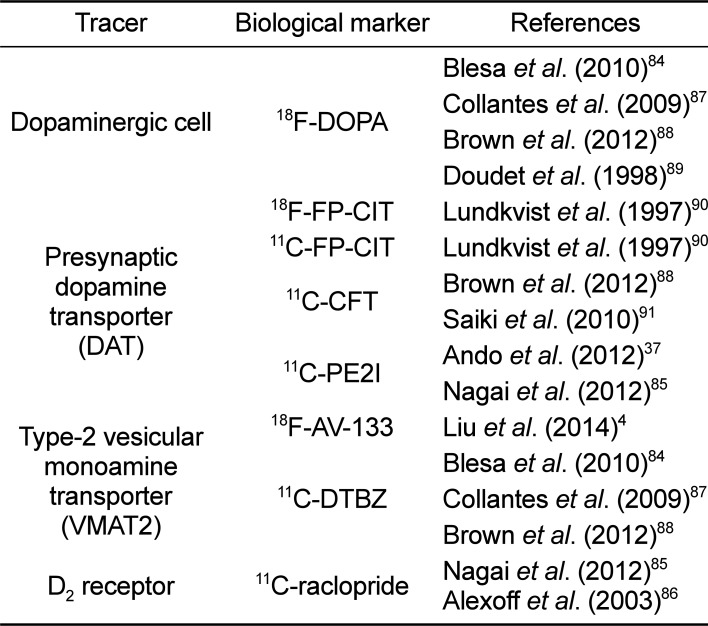
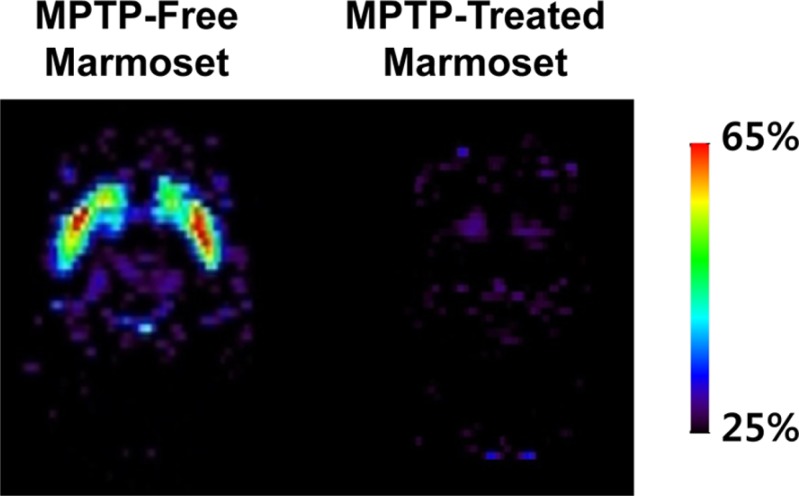
PET imaging is a relatively non-invasive neuroimaging technique that can be used to elucidate the functional changes associated with PD (Figure 4) and to provide diagnostic information by evaluating the integrity of the nigrostriatal DA system [48485]. In particular, a microPET scanner has been designed for the imaging of small-bodied animals, such as the marmoset. Under isoflurane anesthesia, the marmosets should be kept in a stereotaxic unit throughout the scanning process. Tracer compounds labeled with positron-emitting radioisotopes, including 18F-DOPA, 18F-FP-CIT, 11C-FP-CIT, 11C-CFT, 11C-PE2I, 18F-AV-133, 11C-DTBZ, and 11C-raclopride, should be administered to image and measure biochemical processes in vivo (Table 5) [4378485868788899091].
PET studies with 18F-DOPA [84878889] have been used to examine the dysfunction of the nigrostriatal DA system. 11C-raclopride [8586] specifically binds to dopamine D2 receptors. Also, 18F-AV-133 [4] and 11C-DTBZ [848788] target the vesicular monoamine transporter type 2 (VMAT2), which is a vesicular membrane protein that transports monoamines (including DA, norepinephrine, and serotonin) into synaptic vesicles. DATs are located on DA nerve terminals. The DAT-selective ligands 18F-FP-CIT [90], 11C-FP-CIT [90], 11C-CFT [8891], and 11C-PE2I [3785] have been used as a marker of DA nerve degeneration. When DA nerve terminals in the nigrostriatal system degenerate in PD, DAT is not available to bind to these ligands.
The rapid advancement in functional and structural brain imaging has made it feasible to quantify specific changes in brain function within the larger perspective of whole-brain systems [92]. MRI is far more widely available than PET and is the most commonly used system for anatomical imaging of the entire brain in vivo. It can non-invasively acquire functional images showing openings in the BBB, the presence of lesions caused by the Parkinsonian agent [9394]. Marmosets, which are a suitable species for PD research, have been increasingly studied with MRI. Unlike larger monkeys, MRI scans are not needed for the marmoset because a standard frame-based stereotactic apparatus fitted with a small primate head-holder for a variety of neurosurgical procedures is sufficient to perform reproducible intracerebral surgeries [4995]. MR spectroscopy can also be utilized as a non-invasive tool to assess in vivo dynamic changes associated with neurodegeneration and anti-Parkinsonian treatments by measuring the presence and concentration of certain metabolites, especially in MR spectra acquired at high magnetic field strengths and with short echo times [9697].
Biomarkers are needed as indicators for the diagnosis and monitoring of disease progression in PD. DJ-1, a redox-sensitive molecular chaperone protein, appears to be linked to PD with oxidative stress in the mitochondria and nucleus [9]. Also, a pathological sign of PD is the presence of intracellular proteinaceous inclusions, LBs, which are composed mainly of α-synuclein [37]. Consequently, DJ-1 and α-synuclein are considered helpful diagnostic markers for PD in human cerebrospinal fluid as well as in plasma or serum [98] although the use of plasma DJ-1 and α-synuclein as biomarkers is controversial [99]. As tyrosine hydroxylase (TH) catalyses the formation of L-DOPA associated with the biosynthesis of DA [100], immunostaining for TH-positive neurons and DAT have been used to assess nigral DA neuron death after MPTP administration [45]. It has also been reported that significant increases in the pro-inflammatory cytokines IFN-γ and TNF-α in the blood serum samples of MPTP-treated monkeys were observed with ELISA measurements, indicating a critical role in stimulating and maintaining glial cell activation in the SNpc as well as contributions to DA neuronal degeneration and motor impairment Parkinsonism [101].
A number of anti-Parkinsonian drugs, including DA drugs (apomorphine, pramipexole, ropinirole, pergolide, bromocriptine and cabergoline), muscarinic antagonists (benztropine and trihexyphenidyl), monoamine oxidase-B inhibitors (selegiline and rasagiline), and catechol-O-methyltransferase inhibitor (tolcapone), are in current clinical use for PD. These drugs have shown different levels of predictive validity for efficacy depending on the animal models, which reflect different degrees of the pathology and biochemical changes associated with PD [63]. To determine the appropriateness of the model for the discovery of novel treatment for PD symptoms, the similarity of the model with the human disease is especially important. On a basis of the strengths and weaknesses of the respective available models, the researchers should select optimal experimental animal models of PD depending on different target mechanisms to find potential drugs for therapy. In this respect, primate (marmoset) models are particularly useful. More importantly, the fact that the MPTP model possesses the greatest similarity to the clinical features of human PD disease such as tremors, rigidity, akinesia, and postural instability can lead researchers to consider it the most clinically-relevant model for late phase preclinical assessment of a novel treatment [3749]. However, the 6-OHDA model also has an advantage associated with the unilateral lesion to dissociate different symptoms of DA loss [63]. With the growth of biotechnology-derived products, stem cell therapy and gene therapy can be considered potential and novel therapies that provide a more permanent remedy than current drug treatments. Takagi et al. [102] reported that the transplantation of DA neurons generated from monkey ES cells resulted in attenuation of MPTP-induced neurological symptoms in the primate MPTP model for PD as evidenced by behavioral studies and functional imaging. In addition, Kikuchi et al. [103] demonstrated that human induced pluripotent stem cell-derived neural progenitor cells survived for six months as DA neurons in the brain of the primate MPTP model for PD. Lentiviral delivery of glial cell line-derived neurotrophic factor into the striatum and SN has been shown to reverse functional deficits and to prevent nigrostriatal degeneration in the primate MPTP model for PD [104].
In general, research laboratories and pharmaceutical/biotechnology companies follow a sequence of steps for the research and development of treatments related to a variety of important human diseases. An important stage in this process includes the determination of appropriate animal models for predicting the effectiveness of the treatment strategies in clinical trials. Although animal models may not sufficiently reflect the features of the human disease, they can be used to analyze particular aspects and pathogenic mechanisms of the disorder which cannot be fully studied using simple in vitro models. Interest in NHPs is increasing since they are the only relevant species that show high similarity to humans for preclinical assessment prior to clinical trial. At the preclinical stage, use of the marmoset as an experimental model offers several considerable advantages such as smaller size, early sexual maturation, and rapid breeding in pharmaceutical product development, resulting in reduced test material requirements and earlier assessment of product candidates in adults. In particular, the marmoset can be used as an appropriate PD model that reflects various aspects of the human disease as well as an experimental subject for adequate safety or efficacy assessments of the therapeutic treatment, especially with stem cell therapy and gene therapy for PD. Currently, the significant progress in the production of transgenic marmoset models for PD is being made. However, the neurotoxic models are relatively easy to induce parkinsonism in NHPs including the marmosets although the animals with severe symptoms following the systemic or local administration of the neurotoxins need the intensive care with artificial feedings. By providing an overview of the models, methods, and animal care procedures associated with PD research, this article may help researchers with the selection of appropriate animal PD models depending on the specific objectives and aims of their study.
Acknowledgments
This research was supported by grants from the Ministry of Food and Drug Safety (11182MFDS613, 13182MFDS664) and a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI14C2289) in Korea.
References
1. Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998; 339(15):1044–1053. PMID: 9761807.
2. Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003; 39(6):889–909. PMID: 12971891.
3. Yuan H, Zhang ZW, Liang LW, Shen Q, Wang XD, Ren SM, Ma HJ, Jiao SJ, Liu P. Treatment strategies for Parkinson's disease. Neurosci Bull. 2010; 26(1):66–76. PMID: 20101274.

4. Liu Y, Yue F, Tang R, Tao G, Pan X, Zhu L, Kung HF, Chan P. Progressive loss of striatal dopamine terminals in MPTP-induced acute parkinsonism in cynomolgus monkeys using vesicular monoamine transporter type 2 PET imaging ([(18)F]AV-133). Neurosci Bull. 2014; 30(3):409–416. PMID: 24061965.

5. Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009; 8(4):382–397. PMID: 19296921.

6. Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. In : Yahr M, Bergmann KJ, editors. Parkinson's disease. New York: Raven Press;1987. p. 19–34.
7. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004; 318(1):121–134. PMID: 15338272.

8. Le W, Sayana P, Jankovic J. Animal models of Parkinson's disease: a gateway to therapeutics? Neurotherapeutics. 2014; 11(1):92–110. PMID: 24158912.

9. Blandini F, Armentero MT. Animal models of Parkinson's disease. FEBS J. 2012; 279(7):1156–1166. PMID: 22251459.

10. Tieu K. A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harb Perspect Med. 2011; 1(1):a009316. PMID: 22229125.

11. Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med. 2012; 17(6):336–340. PMID: 22871417.

12. Orsi A, Rees D, Andreini I, Venturella S, Cinelli S, Oberto G. Overview of the marmoset as a model in nonclinical development of pharmaceutical products. Regul Toxicol Pharmacol. 2011; 59(1):19–27. PMID: 21156195.

13. Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus). Comp Med. 2003; 53(4):364–368. PMID: 14524412.
14. Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003; 53(4):339–350. PMID: 14524409.
15. Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003; 53(4):383–392. PMID: 14524414.
16. Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003; 100(7):4078–4083. PMID: 12642658.

17. Bezard E, Jaber M, Gonon F, Boireau A, Bloch B, Gross CE. Adaptive changes in the nigrostriatal pathway in response to increased 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration in the mouse. Eur J Neurosci. 2000; 12(8):2892–2900. PMID: 10971632.

18. Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998; 152(2):259–277. PMID: 9710526.

19. Schneider JS, Schroeder JA, Rothblat DS. Differential recovery of sensorimotor function in GM1 ganglioside-treated vs. spontaneously recovered MPTP-treated cats: partial striatal dopaminergic reinnervation vs. neurochemical compensation. Brain Res. 1998; 813(1):82–87. PMID: 9824674.

20. Mikkelsen M, Møller A, Jensen LH, Pedersen A, Harajehi JB, Pakkenberg H. MPTP-induced Parkinsonism in minipigs: A behavioral, biochemical, and histological study. Neurotoxicol Teratol. 1999; 21(2):169–175. PMID: 10192277.
21. Baskin DS, Browning JL, Widmayer MA, Zhu ZQ, Grossman RG. Development of a model for Parkinson's disease in sheep using unilateral intracarotid injection of MPTP via slow continuous infusion. Life Sci. 1994; 54(7):471–479. PMID: 8309350.

22. Jenner P, Rupniak NM, Rose S, Kelly E, Kilpatrick G, Lees A, Marsden CD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridineinduced parkinsonism in the common marmoset. Neurosci Lett. 1984; 50(1-3):85–90. PMID: 6436758.

23. Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM. Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson's disease. Can J Neurol Sci. 2010; 37(1):86–95. PMID: 20169779.

24. Philippens IH, Wubben JA, Finsen B, 't Hart BA. Oral treatment with the NADPH oxidase antagonist apocynin mitigates clinical and pathological features of parkinsonism in the MPTP marmoset model. J Neuroimmune Pharmacol. 2013; 8(3):715–726. PMID: 23504289.

25. Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, Okano H, Huttner WB, Borrell V. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset Callithrix jacchus. Cereb Cortex. 2012; 22(2):469–481. PMID: 22114084.

26. Smith D, Trennery P, Farningham D, Klapwijk J. The selection of marmoset monkeys (Callithrix jacchus) in pharmaceutical toxicology. Lab Anim. 2001; 35(2):117–130. PMID: 11315160.

27. Dell'Mour V, Range F, Huber L. Social learning and mother's behavior in manipulative tasks in infant marmosets. Am J Primatol. 2009; 71(6):503–509. PMID: 19319974.
28. Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease. J Neurosci. 2005; 25(4):769–777. PMID: 15673656.

29. Hansard MJ, Smith LA, Jackson MJ, Cheetham SC, Jenner P. Dopamine, but not norepinephrine or serotonin, reuptake inhibition reverses motor deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Pharmacol Exp Ther. 2002; 303(3):952–958. PMID: 12438514.

30. Annett LE, Torres EM, Clarke DJ, Ishida Y, Barker RA, Ridley RM, Baker HF, Dunnett SB. Survival of nigral grafts within the striatum of marmosets with 6-OHDA lesions depends critically on donor embryo age. Cell Transplant. 1997; 6(6):557–569. PMID: 9440865.

31. Bezard E, Przedborski S. A tale on animal models of Parkinson's disease. Mov Disord. 2011; 26(6):993–1002. PMID: 21626544.

32. Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, Baker H, Ridley RM, Kirik D. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007; 130(Pt 3):799–815. PMID: 17303591.

33. Sasaki E. Prospects for genetically modified non-human primate models, including the common marmoset. Neurosci Res. 2015; 93:110–115. PMID: 25683291.

34. Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009; 459(7246):523–527. PMID: 19478777.

35. Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010; 66(5):646–661. PMID: 20547124.

36. Santana M, Palmér T, Simplício H, Fuentes R, Petersson P. Characterization of long-term motor deficits in the 6-OHDA model of Parkinson's disease in the common marmoset. Behav Brain Res. 2015; 290:90–101. PMID: 25934488.

37. Ando K, Obayashi S, Nagai Y, Oh-Nishi A, Minamimoto T, Higuchi M, Inoue T, Itoh T, Suhara T. PET analysis of dopaminergic neurodegeneration in relation to immobility in the MPTP-treated common marmoset, a model for Parkinson's disease. PLoS One. 2012; 7(10):e46371. PMID: 23056291.

38. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983; 219(4587):979–980. PMID: 6823561.

39. Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979; 1(3):249–254. PMID: 298352.

40. Bezard E, Imbert C, Gross CE. Experimental models of Parkinson's disease: from the static to the dynamic. Rev Neurosci. 1998; 9(2):71–90. PMID: 9711900.

41. Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001; 76(5):1265–1274. PMID: 11238711.

42. Chen MK, Kuwabara H, Zhou Y, Adams RJ, Brasiæ JR, McGlothan JL, Verina T, Burton NC, Alexander M, Kumar A, Wong DF, Guilarte TR. VMAT2 and dopamine neuron loss in a primate model of Parkinson's disease. J Neurochem. 2008; 105(1):78–90. PMID: 17988241.

43. Bergman H, Raz A, Feingold A, Nini A, Nelken I, Hansel D, Ben-Pazi H, Reches A. Physiology of MPTP tremor. Mov Disord. 1998; 13(Suppl 3):29–34. PMID: 9827591.

44. Forno LS, DeLanney LE, Irwin I, Langston JW. Similarities and differences between MPTP-induced parkinsonsim and Parkinson's disease. Neuropathologic considerations. Adv Neurol. 1993; 60:600–608. PMID: 8380528.
45. Serra PA, Pluchino S, Marchetti B, Desole MS, Miele E. The MPTP mouse model: cues on DA release and neural stem cell restorative role. Parkinsonism Relat Disord. 2008; 14(Suppl 2):S189–S193. PMID: 18579428.

46. Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem. 1987; 48(6):1787–1793. PMID: 3106573.

47. Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985; 36(26):2503–2508. PMID: 2861548.

48. Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol. 1986; 20(4):449–455. PMID: 3024555.

49. Eslamboli A. Marmoset monkey models of Parkinson's disease: which model, when and why? Brain Res Bull. 2005; 68(3):140–149. PMID: 16325013.

50. Fox SH, Henry B, Hill M, Crossman A, Brotchie J. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov Disord. 2002; 17(6):1180–1187. PMID: 12465055.

51. Hansard MJ, Smith LA, Jackson MJ, Cheetham SC, Jenner P. Dopamine reuptake inhibition and failure to evoke dyskinesia in MPTP-treated primates. Eur J Pharmacol. 2002; 451(2):157–160. PMID: 12231385.

52. van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, Brotchie JM. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J. 2005; 19(9):1140–1142. PMID: 15894565.
53. Rose S, Nomoto M, Jackson EA, Gibb WR, Jaehnig P, Jenner P, Marsden CD. Age-related effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment of common marmosets. Eur J Pharmacol. 1993; 230(2):177–185. PMID: 8422900.

54. Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007; 2(1):141–151. PMID: 17401348.

55. Yang SC, Markey SP, Bankiewicz KS, London WT, Lunn G. Recommended safe practices for using the neurotoxin MPTP in animal experiments. Lab Anim Sci. 1988; 38(5):563–567. PMID: 3264039.
56. Tranzer JP, Thoenen H. Selective destruction of adrenergic nerve terminals by chemical analogues of 6-hydroxydopamine. Experientia. 1973; 29(3):314–315. PMID: 4708713.

57. Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997; 50:55–66. PMID: 9120425.

58. Eslamboli A, Baker HF, Ridley RM, Annett LE. Sensorimotor deficits in a unilateral intrastriatal 6-OHDA partial lesion model of Parkinson's disease in marmoset monkeys. Exp Neurol. 2003; 183(2):418–429. PMID: 14552882.

59. Annett LE, Torres EM, Ridley RM, Baker HF, Dunnett SB. A comparison of the behavioural effects of embryonic nigral grafts in the caudate nucleus and in the putamen of marmosets with unilateral 6-OHDA lesions. Exp Brain Res. 1995; 103(3):355–371. PMID: 7789442.

60. Henderson JM, Stanic D, Tomas D, Patch J, Horne MK, Bourke D, Finkelstein DI. Postural changes after lesions of the substantia nigra pars reticulata in hemiparkinsonian monkeys. Behav Brain Res. 2005; 160(2):267–276. PMID: 15863223.

61. Svenningsson P, Arts J, Gunne L, Andren PE. Acute and repeated treatment with L-DOPA increase c-jun expression in the 6-hydroxydopamine-lesioned forebrain of rats and common marmosets. Brain Res. 2002; 955(1-2):8–15. PMID: 12419516.

62. Garea-Rodríguez E, Schlumbohm C, Czéh B, König J, Helms G, Heckmann C, Meller B, Meller J, Fuchs E. Visualizing dopamine transporter integrity with iodine-123-FP-CIT SPECT in combination with high resolution MRI in the brain of the common marmoset monkey. J Neurosci Methods. 2012; 210(2):195–201. PMID: 22827895.

63. Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011; 164(4):1357–1391. PMID: 21486284.

64. Annett LE, Rogers DC, Hernandez TD, Dunnett SB. Behavioural analysis of unilateral monoamine depletion in the marmoset. Brain. 1992; 115(Pt 3):825–856. PMID: 1352726.

65. Mitchell IJ, Hughes N, Carroll CB, Brotchie JM. Reversal of parkinsonian symptoms by intrastriatal and systemic manipulations of excitatory amino acid and dopamine transmission in the bilateral 6-OHDA lesioned marmoset. Behav Pharmacol. 1995; 6(5-6):492–507. PMID: 11224357.

66. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000; 3(12):1301–1306. PMID: 11100151.

67. Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009; 34(2):279–290. PMID: 19385059.

68. Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002; 136(1):317–324. PMID: 12385818.

69. Alam M, Schmidt WJ. L-DOPA reverses the hypokinetic behaviour and rigidity in rotenone-treated rats. Behav Brain Res. 2004; 153(2):439–446. PMID: 15265640.

70. Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006; 22(2):404–420. PMID: 16439141.
71. Emborg ME. Nonhuman primate models of Parkinson's disease. ILAR J. 2007; 48(4):339–355. PMID: 17712221.

72. Ando K, Maeda J, Inaji M, Okauchi T, Obayashi S, Higuchi M, Suhara T, Tanioka Y. Neurobehavioral protection by single dose l-deprenyl against MPTP-induced parkinsonism in common marmosets. Psychopharmacology (Berl). 2008; 195(4):509–516. PMID: 17879087.

73. Pearce RK, Jackson M, Britton DR, Shiosaki K, Jenner P, Marsden CD. Actions of the D1 agonists A-77636 and A-86929 on locomotion and dyskinesia in MPTP-treated L-dopa-primed common marmosets. Psychopharmacology (Berl). 1999; 142(1):51–60. PMID: 10102782.
74. Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994; 14(5 Pt 1):2531–2544. PMID: 8182426.

75. Verhave PS, Vanwersch RA, van Helden HP, Smit AB, Philippens IH. Two new test methods to quantify motor deficits in a marmoset model for Parkinson's disease. Behav Brain Res. 2009; 200(1):214–219. PMID: 19378465.

76. Przybyszewski AW, Sosale S, Chaudhuri A. Quantification of three-dimensional exploration in the cylinder test by the common marmoset (Callithrix jacchus). Behav Brain Res. 2006; 170(1):62–70. PMID: 16530859.

77. Palmér T, Tamtè M, Halje P, Enqvist O, Petersson P. A system for automated tracking of motor components in neurophysiological research. J Neurosci Methods. 2012; 205(2):334–344. PMID: 22306061.

78. Philippens IH, Melchers BP, Roeling TA, Bruijnzeel PL. Behavioral test systems in marmoset monkeys. Behav Res Methods Instrum Comput. 2000; 32(1):173–179. PMID: 10758675.

79. Annett LE, Martel FL, Rogers DC, Ridley RM, Baker HF, Dunnett SB. Behavioral assessment of the effects of embryonic nigral grafts in marmosets with unilateral 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 1994; 125(2):228–246. PMID: 7906227.

80. Henderson JM, Annett LE, Torres EM, Dunnett SB. Behavioural effects of subthalamic nucleus lesions in the hemiparkinsonian marmoset (Callithrix jacchus). Eur J Neurosci. 1998; 10(2):689–698. PMID: 9749730.

81. Yamane J, Nakamura M, Iwanami A, Sakaguchi M, Katoh H, Yamada M, Momoshima S, Miyao S, Ishii K, Tamaoki N, Nomura T, Okano HJ, Kanemura Y, Toyama Y, Okano H. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010; 88(7):1394–1405. PMID: 20091712.

82. Montoya CP, Astell S, Dunnett SB. Effects of nigral and striatal grafts on skilled forelimb use in the rat. Prog Brain Res. 1990; 82:459–466. PMID: 2127111.
83. Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The "staircase test": a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991; 36(2-3):219–228. PMID: 2062117.

84. Blesa J, Juri C, Collantes M, Peñuelas I, Prieto E, Iglesias E, Martí-Climent J, Arbizu J, Zubieta JL, Rodríguez-Oroz MC, García-García D, Richter JA, Cavada C, Obeso JA. Progression of dopaminergic depletion in a model of MPTP-induced Parkinsonism in non-human primates. An (18)F-DOPA and (11)C-DTBZ PET study. Neurobiol Dis. 2010; 38(3):456–463. PMID: 20304066.

85. Nagai Y, Minamimoto T, Ando K, Obayashi S, Ito H, Ito N, Suhara T. Correlation between decreased motor activity and dopaminergic degeneration in the ventrolateral putamen in monkeys receiving repeated MPTP administrations: a positron emission tomography study. Neurosci Res. 2012; 73(1):61–67. PMID: 22374309.

86. Alexoff DL, Vaska P, Marsteller D, Gerasimov T, Li J, Logan J, Fowler JS, Taintor NB, Thanos PK, Volkow ND. Reproducibility of 11C-raclopride binding in the rat brain measured with the microPET R4: effects of scatter correction and tracer specific activity. J Nucl Med. 2003; 44(5):815–822. PMID: 12732684.
87. Collantes M, Prieto E, Peñuelas I, Blesa J, Juri C, Martí-Climent JM, Quincoces G, Arbizu J, Riverol M, Zubieta JL, Rodriguez-Oroz MC, Luquin MR, Richter JA, Obeso JA. New MRI, 18F-DOPA and 11C-(+)-alpha-dihydrotetrabenazine templates for Macaca fascicularis neuroimaging: advantages to improve PET quantification. Neuroimage. 2009; 47(2):533–539. PMID: 19422919.
88. Brown CA, Campbell MC, Karimi M, Tabbal SD, Loftin SK, Tian LL, Moerlein SM, Perlmutter JS. Dopamine pathway loss in nucleus accumbens and ventral tegmental area predicts apathetic behavior in MPTP-lesioned monkeys. Exp Neurol. 2012; 236(1):190–197. PMID: 22579525.

89. Doudet DJ, Chan GL, Holden JE, McGeer EG, Aigner TA, Wyatt RJ, Ruth TJ. 6-[18F]Fluoro-L-DOPA PET studies of the turnover of dopamine in MPTP-induced parkinsonism in monkeys. Synapse. 1998; 29(3):225–232. PMID: 9635892.

90. Lundkvist C, Halldin C, Ginovart N, Swahn CG, Farde L. [18F] beta-CIT-FP is superior to [11C] beta-CIT-FP for quantitation of the dopamine transporter. Nucl Med Biol. 1997; 24(7):621–627. PMID: 9352532.
91. Saiki H, Hayashi T, Takahashi R, Takahashi J. Objective and quantitative evaluation of motor function in a monkey model of Parkinson's disease. J Neurosci Methods. 2010; 190(2):198–204. PMID: 20488205.

92. Schultz-Darken NJ. Sample collection and restraint techniques used for common marmosets (Callithrix jacchus). Comp Med. 2003; 53(4):360–363. PMID: 14524411.
93. Pavese N, Brooks DJ. Imaging neurodegeneration in Parkinson's disease. Biochim Biophys Acta. 2009; 1792(7):722–729. PMID: 18992326.

94. Miletich RS, Bankiewicz KS, Quarantelli M, Plunkett RJ, Frank J, Kopin IJ, Di Chiro G. MRI detects acute degeneration of the nigrostriatal dopamine system after MPTP exposure in hemiparkinsonian monkeys. Ann Neurol. 1994; 35(6):689–697. PMID: 8210225.

95. Stephan H, Baron G, Schwerdtfeger WK. The brain of the common marmoset (Callithrix jacchus): a Stereotaxic Atlas. Berlin: Springer-Verlag;1980.
96. Brownell AL, Jenkins BG, Elmaleh DR, Deacon TW, Spealman RD, Isacson O. Combined PET/MRS brain studies show dynamic and long-term physiological changes in a primate model of Parkinson disease. Nat Med. 1998; 4(11):1308–1312. PMID: 9809556.

97. Gröger A, Kolb R, Schäfer R, Klose U. Dopamine reduction in the substantia nigra of Parkinson's disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS One. 2014; 9(1):e84081. PMID: 24416192.

98. Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010; 133(Pt 3):713–726. PMID: 20157014.
99. Shi M, Zabetian CP, Hancock AM, Ginghina C, Hong Z, Yearout D, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Leverenz JB, Zhang J. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson's disease. Neurosci Lett. 2010; 480(1):78–82. PMID: 20540987.

100. Haavik J, Toska K. Tyrosine hydroxylase and Parkinson's disease. Mol Neurobiol. 1998; 16(3):285–309. PMID: 9626667.

101. Barcia C, Ros CM, Annese V, Gómez A, Ros-Bernal F, Aguado-Yera D, Martínez-Pagán ME, de Pablos V, Fernandez-Villalba E, Herrero MT. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death Dis. 2011; 2:e142. PMID: 21472005.

102. Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005; 115(1):102–109. PMID: 15630449.

103. Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson's disease. J Parkinsons Dis. 2011; 1(4):395–412. PMID: 23933658.

104. Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000; 290(5492):767–773. PMID: 11052933.

Figure 1
The marmoset in a housing facility. A marmoset (A) and examples of a cage (B), an enclosed cage under negative-pressure (C), and an intensive care unit with tightly controlled temperature, humidity, and oxygen concentration (D).
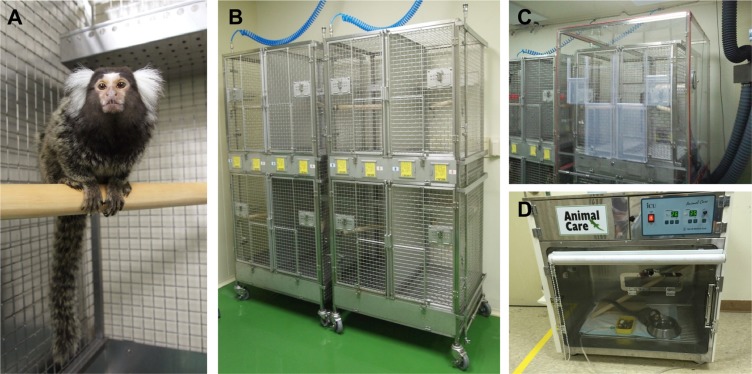
Table 1
MPTP regimens for the common marmoset in PD model
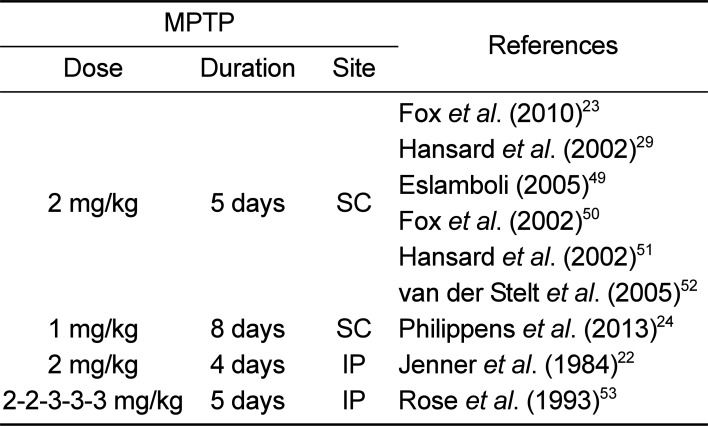
| MPTP | References | ||
|---|---|---|---|
| Dose | Duration | Site | |
| 2 mg/kg | 5 days | SC | Fox et al. (2010)23 |
| Hansard et al. (2002)29 | |||
| Eslamboli (2005)49 | |||
| Fox et al. (2002)50 | |||
| Hansard et al. (2002)51 | |||
| van der Stelt et al. (2005)52 | |||
| 1 mg/kg | 8 days | SC | Philippens et al. (2013)24 |
| 2 mg/kg | 4 days | IP | Jenner et al. (1984)22 |
| 2-2-3-3-3 mg/kg | 5 days | IP | Rose et al. (1993)53 |
Table 2
6-OHDA regimens for the common marmoset in PD model
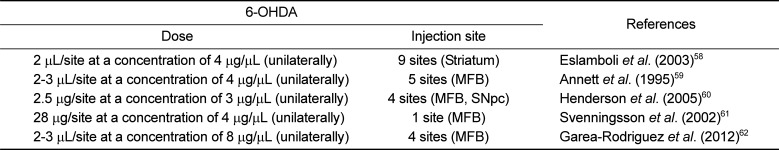
| 6-OHDA | References | |
|---|---|---|
| Dose | Injection site | |
| 2 µL/site at a concentration of 4 µg/µL (unilaterally) | 9 sites (Striatum) | Eslamboli et al. (2003)58 |
| 2-3 µL/site at a concentration of 4 µg/µL (unilaterally) | 5 sites (MFB) | Annett et al. (1995)59 |
| 2.5 µg/site at a concentration of 3 µg/µL (unilaterally) | 4 sites (MFB, SNpc) | Henderson et al. (2005)60 |
| 28 µg/site at a concentration of 4 µg/µL (unilaterally) | 1 site (MFB) | Svenningsson et al. (2002)61 |
| 2-3 µL/site at a concentration of 8 µg/µL (unilaterally) | 4 sites (MFB) | Garea-Rodriguez et al. (2012)62 |
Table 3
Overview of several disability examinations in PD model
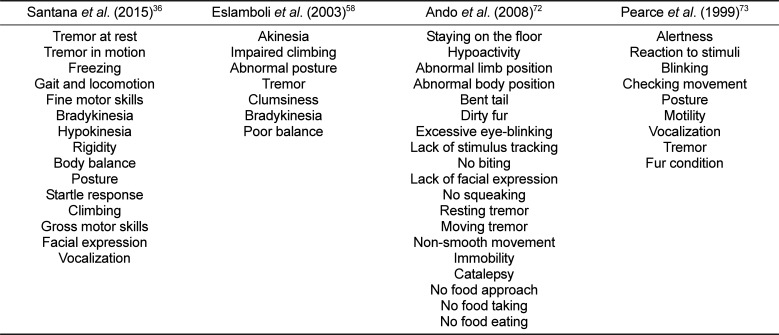
| Santana et al. (2015)36 | Eslamboli et al. (2003)58 | Ando et al. (2008)72 | Pearce et al. (1999)73 |
|---|---|---|---|
| Tremor at rest | Akinesia | Staying on the floor | Alertness |
| Tremor in motion | Impaired climbing | Hypoactivity | Reaction to stimuli |
| Freezing | Abnormal posture | Abnormal limb position | Blinking |
| Gait and locomotion | Tremor | Abnormal body position | Checking movement |
| Fine motor skills | Clumsiness | Bent tail | Posture |
| Bradykinesia | Bradykinesia | Dirty fur | Motility |
| Hypokinesia | Poor balance | Excessive eye-blinking | Vocalization |
| Rigidity | Lack of stimulus tracking | Tremor | |
| Body balance | No biting | Fur condition | |
| Posture | Lack of facial expression | ||
| Startle response | No squeaking | ||
| Climbing | Resting tremor | ||
| Gross motor skills | Moving tremor | ||
| Facial expression | Non-smooth movement | ||
| Vocalization | Immobility | ||
| Catalepsy | |||
| No food approach | |||
| No food taking | |||
| No food eating |
Table 4
Overview of several behavioral tests in PD model
| Behavioral test | References |
|---|---|
| Locomotor activity assessment | Roberts et al. (1994)74 |
| Object retrieval task | Eslamboli et al. (2003)58 |
| Cylinder test | Przybyszewki et al. (2006)76 |
| Tower test | Verhave et al. (2009)75 |
| Palmer et al. (2012)77 | |
| Bungalow test | Philippens et al. (2000)78 |
| Hand-eye coordination test | Philippens et al. (2000)78 |
| Fear-potentiated startle response | Philippens et al. (2000)78 |
| Rotation test | Annett et al. (1994)79 |
| Henderson et al. (1998)80 | |
| Conveyor belt test | Annett et al. (1994)79 |
| Adhesive labels | Annett et al. (1994)79 |
| Reaching into tubes | Annett et al. (1994)79 |
| Henderson et al. (1998)80 | |
| Staircase | Eslamboli et al. (2003)58 |
| Henderson et al. (1998)80 | |
| Montoya et al. (1990)82 | |
| Montoya et al. (1991)83 | |
| Hourglass test | Verhave et al. (2009)75 |
| Bar grip power | Yamane et al. (2010)81 |
| Treadmill test | Yamane et al. (2010)81 |
Table 5
The tracers for functional PET imaging in PD model
| Tracer | Biological marker | References |
|---|---|---|
| Dopaminergic cell | 18F-DOPA | Blesa et al. (2010)84 |
| Collantes et al. (2009)87 | ||
| Brown et al. (2012)88 | ||
| Doudet et al. (1998)89 | ||
| Presynaptic dopamine transporter (DAT) | 18F-FP-CIT | Lundkvist et al. (1997)90 |
| 11C-FP-CIT | Lundkvist et al. (1997)90 | |
| 11C-CFT | Brown et al. (2012)88 | |
| Saiki et al. (2010)91 | ||
| 11C-PE2I | Ando et al. (2012)37 | |
| Nagai et al. (2012)85 | ||
| Type-2 vesicular monoamine transporter (VMAT2) | 18F-AV-133 | Liu et al. (2014)4 |
| Blesa et al. (2010)84 | ||
| 11C-DTBZ | Collantes et al. (2009)87 | |
| Brown et al. (2012)88 | ||
| D2 receptor | 11C-raclopride | Nagai et al. (2012)85 |
| Alexoff et al. (2003)86 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download