Zinc oxide (ZnO) is a multifunctional inorganic compound due to its unique physical and chemical properties [
1]. ZnO is a white powder that is insoluble in water and is widely used as an additive in numerous materials and products including rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, food, batteries, ferrites, fire retardants, and first-aid tape.
ZnO nanoparticles (NPs) are one of the most commonly used nanomaterials in the industry [
2]. ZnO NPs are used in various product categories because of their unique photocatalytic, electronic, optical, dermatological, and antibacterial properties; however, the most common applications are in sunscreens, baby powder, antidandruff shampoos, and fabric treatments for UV shielding [
34]. ZnO NPs have received more attention due to their putative anticancer properties and for drug delivery [
5]. However, nanomaterials are associated with some problems, including toxicity and their environmental impact. Furthermore, limited information is available about the potential toxicity and mechanisms underlying the toxic effects of ZnO NPs.
Because ZnO NPs are the most commonly utilized nanomaterials in various consumer products, many studies have shown the toxic effects of ZnO NPs in several experimental models [
67]. In particular,
in vivo studies are necessary to investigate the toxic effects of NPs in biological systems. Before evaluating the potential toxicity of NPs, it is important to understand how living organisms are exposed to them. Exposure can occur through the lungs, skin, or digestive system, as shown by a number of
in vivo studies on ZnO NPs nanotoxicity [
8]. For example, ZnO NPs accumulate significantly in the livers of mice after oral administration of 30 nm ZnO NPs for 14 days, resulting in oxidative stress mediated by DNA damage and apoptosis [
9]. Similarly, ZnO NPs impair mitochondria and cell membranes in rat kidneys after oral administration of ZnO NPs for 14 days [
10]. Repeated administration for 28 days through a dermal route decreases the collagen level at the application site [
11]. These results suggest that nanotoxicity of ZnO NPs may be mediated by the production of ROS and oxidative DNA damage based on their
in vitro toxic mechanisms [
12].
The toxicity of NPs depends on their physicochemical properties, such as particle size, particle shape, surface area, and surface charge. Pasupuleti et al. [
13] reported differences in nanotoxicity between nanosized ZnO and microsized ZnO particles after 14-day oral administration to rats. They found that the incidences of lesions in the liver, pancreas, heart, and stomach were higher in rats treated with low doses of NPs than those treated with high doses; however, high doses of the microsized NPs caused more lesions than the low doses. In addition, Ho et al. [
14] found that mass and surface area are effective metrics responsible for ZnO NPs toxicity through inhalation exposure. These results suggest that particle size and dose metrics are key nanotoxicological concepts that need to be considered when evaluating the toxicity of manufactured NPs. Another critical factor for nanotoxicity is surface charge, particularly that of ligands, which modify their surface and affect cellular responses to NPs. However, information regarding the charge effect of NPs on
in vivo toxicity is very limited because of the complexity and dissolution properties of
in vivo systems.
In the present study, we performed a 14-day repeated oral dose toxicity study of positively charged 100 nm ZnO NPs in Sprague-Dawley (SD) rats to determine the potential subacute toxicity and to select treatment doses for a 90-day repeated dose toxicity study.
Twenty-four 5-week-old SD rats of each sex were obtained from a specific pathogen-free colony at Orient Bio (Seoul, Korea) and used after 1 week of quarantine and acclimatization. Two animals per cage were housed in a room maintained at 22±3℃ and a relative humidity of 50±20% with artificial lighting on from 08:00 to 20:00 and 10-15 air exchanges/hour. The animals were provided ultraviolet-sterilized tap water and commercial rodent chow (Cargill Agri Purina Inc., Pyeongtaek, IN, Korea) ad libitum. This study was performed in accordance with the regulations for the care and use of laboratory animals in the Health Care Research Laboratory in the Korea Testing and Research Institute (TBH001172) based on the Animal Protection Act No. 10995.
The test article was 100 nm ZnO NPs, which were purchased from American Elements (Los Angeles, CA, USA). Surface charge was modified with an L-serine coating reagent (for [+] charge) (Sigma-Aldrich, St. Louis, MO, USA, CAS No 56-45-1). L-serine was dissolved in 20mM HEPES buffer to prepare ZnO
AE100(+), and pH was set 6 using 1M Na
2CO
3 solution. The final concentration of sodium citrate and L-serine was 1 w/v% in 20 mM HEPES buffer solution. The test article was suspended in HEPES-serine buffer (Sigma-Aldrich), and dosing solutions were prepared daily before treatment. Before initiating this study, the physicochemical properties of the test article including the primary particle size, morphology, hydrodynamic size, and zeta potential were verified [
1516]. The formulated test articles were freshly homogenized by vortexing before administration. The daily ZnO
AE100(+) application volume (10 mL/kg body weight) was calculated in advance based on the most recently recorded body weight of each individual animal. The test mixture was administered by gavage to rats for 14 days. Vehicle control rats received an equivalent volume of HEPES-serine buffer alone.
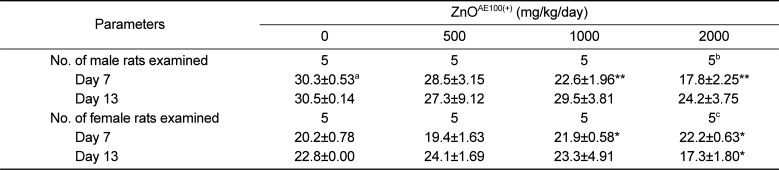
Healthy male and female rats were assigned randomly to four experimental groups (n=5 males and 5 females per group): three treatment groups receiving 500, 1,000, and 2,000 mg/kg/day ZnOAE100(+), and a vehicle control group. A vehicle control group was added to determine the effects of the vehicle. All animals were observed daily throughout the study period for any clinical signs of toxicity and mortality. The body weight of each rat was measured at the beginning of treatment and once per week thereafter during exposure. Feed consumption was recorded weekly after the treatment start date. Consumption was calculated from the difference between the supplied amount and the remaining amount measured the next day.
Necropsies were performed on dead animals, which were found immediately. After a blood sample was collected under deep anesthesia with isoflurane, the animal was killed by exsanguination. The external surface, all orifices, and all organs in the cranial, thoracic, and abdominal cavities and their contents were examined. After necropsy, the absolute and relative (organ-to-body-weight ratio) weights of the major organs were measured in all survivors when they were killed. The major organs included the liver, kidney, spleen, brain, lung, and heart.
The animals were fasted overnight before necropsy. Blood was collected from the abdominal aorta under anesthesia with isoflurane. We collected 3 mL of blood in a CBC bottle (ethylenediaminetetraacetic acid 3 K; Becton, Dickinson and Co., Franklin Lakes, NJ, USA) and analyzed the blood with an autohematoanalyzer (ADVIA120E; Siemens AG, Erfurt, Germany). Additional blood was transferred to Vacutainer tubes (sodium citrate 9NC; Becton, Dickinson and Co.) and centrifuged at 3,000 rpm for 10 min. The plasma was separated and used to determine clotting time with a coagulometer (ACL 7000; Instrumentation Laboratory, Bedford, MA, USA). The hematological parameters assessed were: red blood cells (RBC), hemoglobin concentration (HB), hematocrit (HCT), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), reticulocyte (RET), platelet (PLT), prothrombin time (PT), activated partial thromboplastin time (APTT), white blood cells (WBC), and differential leukocytes (neutrophils, lymphocytes, monocytes, eosinophils, and basophils).
Blood was allowed to clot for 1 hour at room temperature and was centrifuged at 3,000 rpm for 10 min to collect serum for biochemical analyses using an automatic serum analyzer (HITACHI 7060; Hitachi Co., Tokyo, Japan) and an electrolyte analyzer (EasyLyte PLUS Na/K/Cl Analyzer; Medica Corp, Bedford, MA, USA). We analyzed aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CRTN), glucose (GLU), total cholesterol (T-CHO), total bilirubin (T-BIL), total protein (TP), albumin (ALB), the albumin/globulin (A/G) ratio, creatine kinase (CK), triglycerides (TG), calcium (Ca), inorganic phosphate (IP), sodium (Na), potassium (K), and chloride (Cl) levels.
The internal organs from all animals were collected at necropsy and fixed in 10% neutral buffered formalin. The testis and epididymis were fixed in Bouin's solution, and the eyes were fixed in Davidson's solution. A histopathological examination was performed on mice from all groups. The internal organs examined were the brain, heart, liver, spleen, kidney, lung, stomach, eye, and pancreas.
Data on body weight, feed consumption, hematology, serum biochemistry, and organ weights were analyzed for homogeneity of variance using Levene's test. One-way analysis of variance was performed to evaluate the differences. If the variance was homogeneous and a significant difference was confirmed, Scheffé's multiple comparison test was performed as a post-hoc test. If the variance was not homogeneous, the data were analyzed using Dunnett's T3 test. All analyses were performed using SPSS ver. 10.0 software (IBM Corp., Armonk, NY, USA).
As ZnO NPs are used extensively in various commercial applications, they have become a significant source of intended and unintended human exposure [
12]. Although some information on the potential toxicity of ZnO NPs has been accumulated, it is necessary to understand more about other possible ZnO NPs toxicities. We investigated the potential subacute toxicity of positively charged 100 nm ZnO
AE100(+) in a 14-day repeated oral administration to SD rats at doses of 0, 500, 1,000, and 2,000 mg/kg/day to determine the no-observed-adverse-effect level (NOAEL), the effects on target organs, and to select treatment doses for a 90-day repeated dose toxicity study. Our results showed that daily oral administration of ZnO
AE100(+) for 14 days at ≥500 mg/kg/day caused significant changes in clinical signs, body weight, feed consumption, necropsy findings, organ weights, hematology, serum biochemistry, and histopathology in the rats.
Treatment-related clinical signs, as evidenced by dose-dependent increases in the incidence and severity of salivation, abdominal distension, fur loss, and weakness were observed in males of all treated groups and females in the 2,000 mg/kg group (data not shown). Of all adverse clinical signs observed, salivation and abdominal distension probably resulted from irritation of the oral mucosa and gastrointestinal track by ZnO
AE100(+). Salivation is often observed in gavage studies and may have been a reaction to the taste or mucosal irritation of the test article [
17181920]. Fur loss and weakness were indications of stress and/or malnutrition induced by ZnO
AE100(+), which is supported by the decreases in body weight and feed consumption. One treated male died on Day 6 and one treated female died on Day 14 in the 2,000 mg/kg group. The animal deaths observed in both sexes in the high dose group may have resulted from the overt systemic toxicity of ZnO
AE100(+). The necropsy findings included edematous and a yellowish color change in the intestines and a swollen stomach filled with the test article, indicating that the gastrointestinal tract is a major target organ of ZnO
AE100(+) in rats.
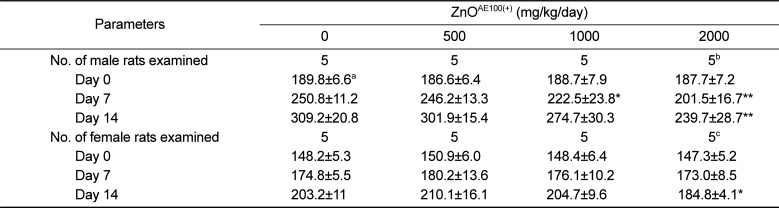
As shown in
Tables 1 and
2, the suppressed body weight gain observed in the 1,000 and 2,000 mg/kg groups was attributed to the test article and was consistent with the increased incidence of clinical signs and decreased feed consumption observed in the groups. These findings are clear indications of the general toxicity induced by ZnO
AE100(+) and suggest that oral administration of ZnO
AE100(+) results in mild anorexia, followed by suppressed of body weight gain in rats.
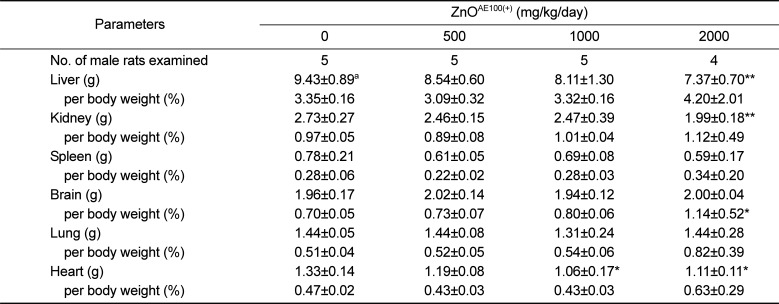
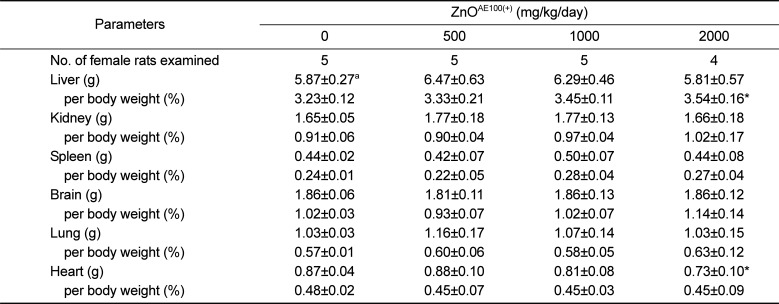
Body and organ weights are sensitive indicators of potentially toxic articles in general toxicity studies [
212223]. As described above, repeated oral dosing of the test article significantly suppressed body weight gain in males administered ≥1,000mg/kg and females administered 2,000 mg/kg ZnO
AE100(+). The suppressed body weight gain affected the absolute weight of some organs, such as the liver, kidney, and heart, or the relative organ to body weights, such as the brain and liver (
Tables 3 and
4). Moreover, no corresponding histopathological changes or accompanying biochemical alterations were detected. Therefore, the weight changes in these organs are of uncertain toxicological significance.
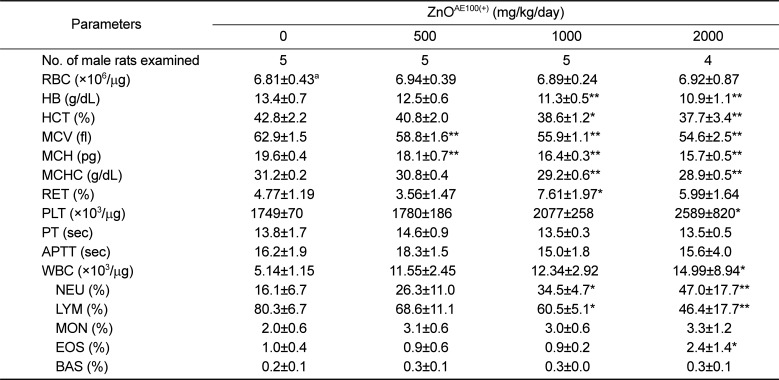
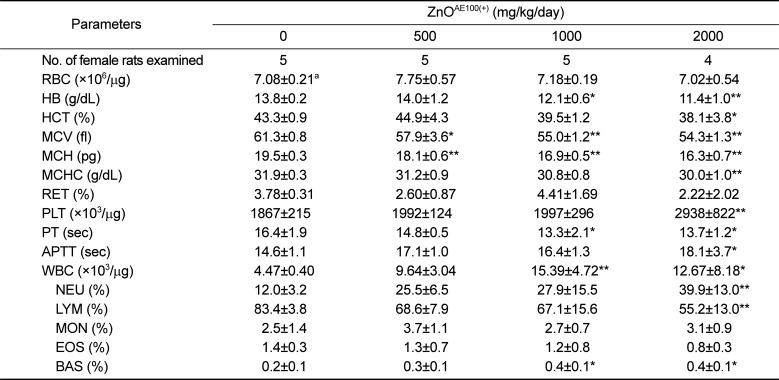
The significant decreases in HB, HCT, MCV, MCH, MCHC, and LYM and the significant increases in PLT, WBC, and NEU observed in both sexes of treated rats were closely related to administration of ZnO
AE100(+) because these changes were remarkable and showed a clear-cut dose-response relationship (
Tables 5 and
6). One study showed that a high zinc diet results in iron deficiency anemia and that this zinc-induced anemia is related to decreases in HB, HCT, MCV, MCH, and MCHC levels [
10], which corresponded with our results. Similarly, a 13-week oral administration of 40 nm ZnO NPs to rats also caused significant changes in anemia-related hematological parameters [
24]. As thrombocytosis frequently accompanies the less severe iron deficiency anemia [
25], the stimulation of platelets may have been related to iron deficiency anemia. The increased WBC and NEU counts and the decreased LYM count observed in the ≥1,000 mg/kg groups were related to ZnO
AE100(+) treatment, as these changes showed a clear-cut dose-response relationship and they were observed in both sexes. The leukocytosis observed in the ≥1,000 mg/kg groups may have resulted from the inflammatory lesions detected in the stomach and pancreas. In contrast, the decrease in PT and increases in RET, APTT, EOS, and BAS observed in treated males or females were incidental and not exposure-related, as these changes did not exhibit a dose-response relationship and were observed in only one sex.
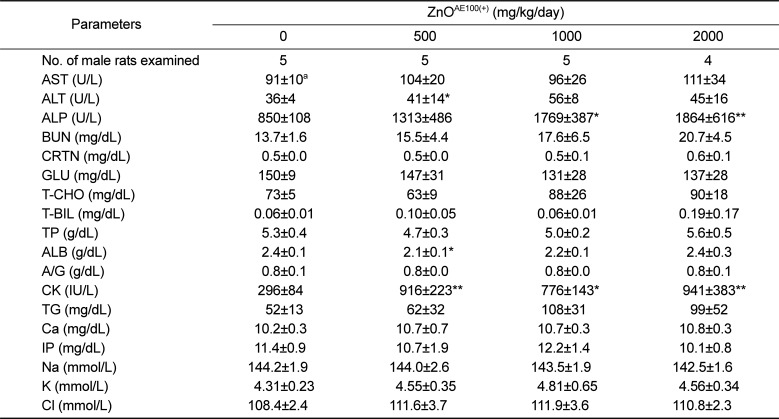
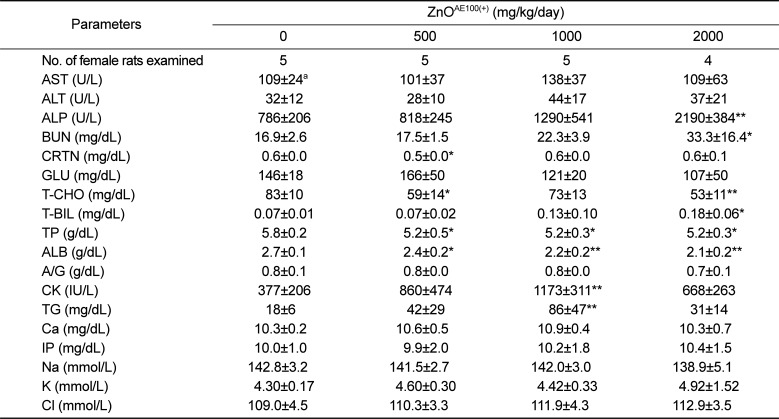
The significant ALP increase observed in the treated males and females was due to the ZnO
AE100(+) treatment because the increase was remarkable and showed a clear-cut dose-response relationship (
Tables 7 and
8). The other significant serum biochemistry findings were incidental because they did not show a dose-response relationship and were unaccompanied by correlated pathological changes.
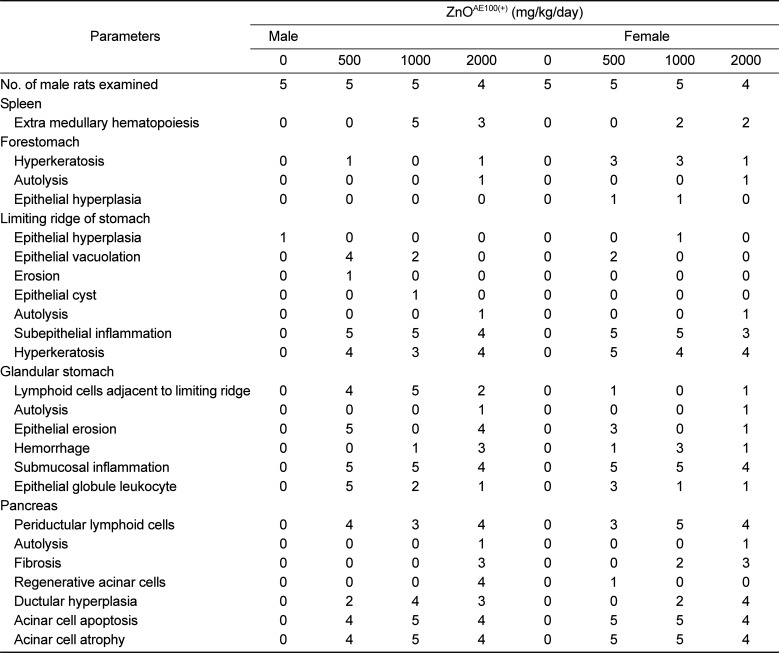
The histopathological examination showed prominent changes in the spleen, stomach, and pancreas (
Table 9). The principal findings included extra-medullary hematopoiesis in the spleen, hyperkeratosis and epithelial hyperplasia in the forestomach, epithelial vacuolation, subepithelial inflammation, and hyperkeratosis in limiting ridge of stomach; lymphoid cells adjacent to the limiting ridge, epithelial erosion, hemorrhage, submucosal inflammation, and epithelial globule leukocytes in the glandular stomach; and periductular lymphoid cells, fibrosis, regenerative acinar cells, ductular hyperplasia, acinar cell apoptosis, and acinar cell atrophy in the pancreas. The dose-dependent increases in the incidence and severity of these findings with increasing dose indicates that these findings were caused by the ZnO
AE100(+) treatment. A number of single and repeated dose toxicity studies have demonstrated that oral administration of 20-120 nm sized ZnO NPs causes pathological damage in the stomach, liver, heart, spleen, and/or pancreas in experimental animals [
6131524]. The other histopathological changes observed in the treatment groups were not treatment-related effects because they occurred at a low incidence and did not exhibit a dose-response relationship. Moreover, these findings have been documented to occur commonly in healthy SD rats [
26272829].
In conclusion, a 14-day repeated oral dosing of ZnO
AE100(+) to rats resulted in significant alterations in clinical signs, body weight, hematology, serum biochemistry, and histopathology at ≥500 mg/kg/day. The target organs were the spleen, stomach, and pancreas. The NOAEL was <500 mg/kg for both sexes because the adverse effects were observed at all doses tested. Although the single and repeated dose toxicities of ZnO NPs have been recently reported [
6131524], the results of this study are expected to provide valuable information on the general toxic effects and target organ toxicity of positively charged 100 nm ZnO NPs at relatively higher doses via repeated oral exposure, which can aid in the process of risk assessment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download