Abstract
The effect of pentoxifylline (PTX) administration on histomorphological parameters of streptozotocin (STZ)-induced type 1 diabetes mellitus (DM) in male rat testes were evaluated. We randomly divided 40 male rats into the following four groups: group 1: control or normal glycemic (NG) rats; group 2 or NG rats that received only normal saline (NS), (NG+NS); group 3 or diabetic rats which were not treated by PTX (DM+vehicle solution (NS)); and group 4 which comprised diabetic rats treated with 50 mg/kg of PTX (DM+PTX). Type 1 DM was induced by intraperitoneal injection of STZ (55 mg/kg). Rats were held for 30 days after which the experimental group received PTX twice daily (25 mg/kg) or NS. After 14 days of treatment by PTX or NS, the left testes from all rats were extracted and prpared for histological study. Apoptotic cells, blood vessel density, and spermatogenesis were evaluated. Data were analyzed by ANOVA test. PTX-treated-diabetic rats showed a significant decrease in number of apoptotic cells and decrease in blood vessel density compared to the DM+NS rats. A significant increase in spermatogenesis was observed in the PTX-treated diabetic group, compared to the DM+NS groups. It was concluded that PTX administration to STZ-induced type 1 DM rats affected apoptotic cell number positively. Moreover, blood vessel density significantly decreased and improvements were observed in spermatogenesis.
Diabetes mellitus (DM) is defined as a group of metabolic disorders that have prolonged elevated blood sugar levels. Today, this disorder is one of the main concerns for human societies around the world [1]. In 2013, according to the International Diabetes Federation, an estimated 381 million people were reported diabetics [2]. The prevalence is rapidly increasing and by 2030 this number is estimated to get double approximately [3]. DM has two main types, types 1 and 2 [2]. Both types either directly or indirectly have a disruptive effect on organs such as the heart, kidneys, eyes, peripheral nerves [3], and male reproductive system, that result in impotency and sterility [456]. DM has harmful effects on accessory reproductive glands, spermatogenesis, ejaculation; in addition decrease testosterone levels, and damage to the tissues and function of testis has been observed [7].
Throughout the years, researchers have attempted to find a cure for DM and decrease its negative effects. Currently, there is a new trend in the preparation and marketing of drugs of choice. Pentoxifylline (PTX) with the chemical name, 1-(5-oxohexyl)-3, 7-dimethylxanthine is a drug currently used as a supplement for treatment of diabetic abnormalities [8]. PTX is a peripheral vasodilator. Its vasodilatative effect is attributed to inhibit the enzyme phosphodiesterase and increase the cyclic adenosine monophosphate (cAMP) concentration in the smooth muscle cells in blood vessels [910]. PTX enhances blood circulation in tissues which causes acceleration of wound healing process in diabetic animal models [11]. In the reproductive field, PTX is usually considered as a treatment of choice for impaired functions and structures of the reproductive system [12]. PTX augments sperm motility and the numbers of spermatozoa in testicular torsion. It has shown to affect the sperm's capacity to undergo acrosome reaction and improves the circulation of testes even in cases of vascular abnormalities [13]. Najar et al. reported that PTX administration increase Sertoli and Leydig cells count in non-diabetic and diabetic rats compared to the control groups. Furthermore, its effect in diabetic rats were significant [14]. PTX probably has a positive effect on histomorphological parameters of diabetic organs such as the reproductive organs compared to non-diabetic organs.
As of now, there are not enough data about PTX on the effect of diabetic in reproductive organs In this study we have aimed to evaluate the histomorphological effects of PTX on the tissues of testes in streptozotocin (STZ)-induced DM rats.
This study used 40 male Wistar rats (Pasteur Institute of Iran) that weighed approximately 250-300 g. Rats were divided into individual cages under controlled conditions (12 h light/dark cycle, temperature: 22-25℃ for approximately one week. During this period all animals had unrestricted access to food and tap water. All procedures were approved by the Medical Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, Iran (protocol no.: 90-1-153-8183). Rats' weight and blood glucose levels were monitored every three days.
The rats were randomly divided into the following four groups. In each group there were ten rats: 1. Control; in other word, normal glycemic (NG) rats which were not treated with PTX, (Sigma Aldrich, St. Louis, MO, USA) or normal saline (NS); 2. NG rats that received only normal saline (NG+NS); 3. DM rats which were treated with NS (DM+vehicle solution,NS); and 4. DM+PTX rats who received 25 mg/kg of PTX twice daily.
In the day zero, groups 3 (DM+NS) and 4 (DM+PTX) after a 12-h fasting received an IP injection of 55 mg/kg STZ (Zanosar Pharmacia and Upjohn Co., Kalamazoo, MI, USA), that was dissolved freshly in sterile water (pH=7.3).
Seven days after STZ injection, blood samples were taken from the veins of rats' tail and analyzed for glucose levels (Biomine, Rightesttm GM300, Biomine Corporation, Switzerland). Rats with blood glucose level greater than 250mg/dL after a week of STZ injection were considered to be type 1 DM.
Rats were held for 30 days from day zero. During this period, rats' blood glucose levels were monitored every three days. If the hyperglycemia was steady, the animals were considered to be type 1 diabetes [11].
PTX, at a dose of 25 mg/kg was intraperitonealy (IP) injected twice daily in the DM rats over 14-day period [11].
At the end of the experiment, all animals were sacrificed. Following an scrotum incision, the right testes were removed and weighed by a fine scale.
Testes were fixed in 10% formalin saline for 72 h. After that they were cut into two equal halves from the middle, and again fixed for 3 h in formalin saline, then embedded in paraffin. We obtained 10, 6 µm histological sections per testes at 30 µm intervals. The sections were stained by hematoxylin and eosin (H&E) for histological study; then evaluated by the TUNEL assay for apoptotic cell.
We randomly chose 30 slides which were evaluated at 100× magnification. The entire interstitial vessel tissue(s) were identified and counted in the fields [16]. Blood vessels and neovasclar were recognized by the presence of blood cells in their lumens.
Apoptosis was studied by the in situ terminal-deoxynucleotidyl-transferase-mediated dUTP digoxigenin nick end labeling (TUNEL) assay. A Dead End Colorimetric TUNEL System Kit (Cat. no. 11 684 817 910; Roche, Germany) was used for apoptotic cell detection. We choose the Zhao et al. protocol [16] for the TUNEL assay. Briefly, 6-µm-thick paraffin-embedded sections were de-paraffinized, rehydrated in a graded alcohol series, and re-treated in trypsin solution at 37℃ for 10 mintues (Roche, Germany) in a microwave. After washing with Phosphate buffer solution (PBS), the specimens were incubated with fluorescein-labeled deoxy-UTP and terminal deoxynucleotidyl transferase at 37℃ for 1 h. Subsequently, converter POD solutions were used in the slides. Diaminobenzidine was used for section staining in a light microscope at the magnification of 400× for apoptotic cell determination. We counted the TUNEL-positive cells in 10 seminiferous tubule cross-sections from the three slides for each rat.
The histological slides were stained with H&E; afterword, examined with a light microscope at 100× magnification to evaluate the spermatogenic activitiy of 30 semineferous tubules in five different fields in each slide. The results were assessed according to the Johnson criteria. In Johnson s score, level 1 to 10 was given to each tubule cross section according to the range from no cell to complete spermatogenesis [17].
Statistical analysis was performed with the SPSS version 16 (SPSS Inc., Chicago, IL, USA). The difference between our four groups in terms of histomorphological parameters was examined by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Results were reported: as mean±SEM and a P-value less than 0.05 was considered significant.
In the 7th day, after STZ injection, the mean body weight in DM+NG rats were 289.2±10.3 g. In the 30th day, after the STZ injection, this weight was 254.9±8.8 g. According to the result, STZ significantly decreased the weight of treated rats (paired student t-test, P=0.02).
Accorded to the results, in the first week of the STZ injections, the mean level of blood glucose was 303.8±31.3 mg/dL. By the end of the experiment, glucose levels increased to 455.2±60.7 mg/dL.
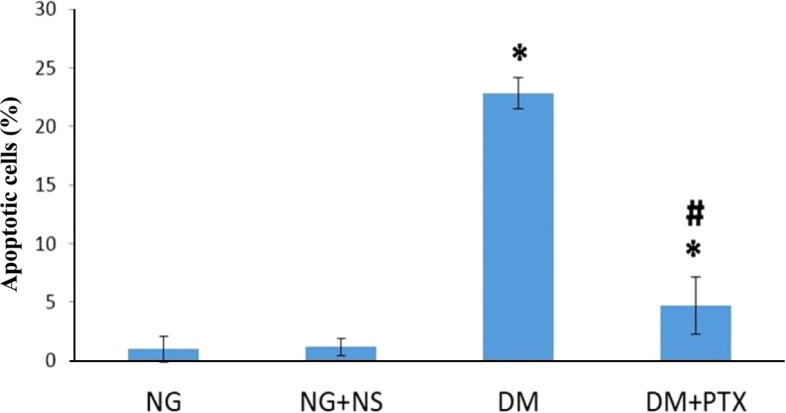
TUNEL-positive cells for each group are shown in Figure 3. In the DM+NS and DM+PTX groups the apoptotic cells significantly increased compared to the NG group (P=0.000; Figure 4). These cells decreased in PTX-DM group compared to the DM+NS group (P=0.000). A significant differenc were observed between the DM+NS group, and the control groups (NG, and NG+NS; P=0.000).
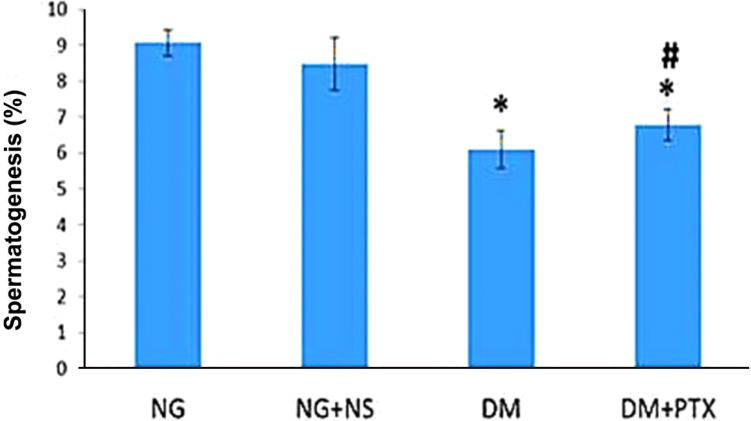
The comparison of spermatogenesis parameters in the groups showed no significant changes in spermatogenesis level to the healthy groups. However, DM+PTX group showed a significant increase in spermatogenesis parameters compared to the DM+NS group (P=0.044). Spermatogenesis parameters in the diabetic groups (DM+NS) and (DM+PTX) showed significant decreases of spermatogenesis compared to the groups NG, and NG+NS (both, P=0.000; Figure 5).
The present study evaluated the histomorphological effects of PTX on the reproductive system of STZ-induced DM rats. TUNEL-positive cells in the testis of diabetic rats significantly increased. The results showed detrimental effects of DM on the spermatogenesis process.
Angiogenesis is characterized by new blood vessel formation from pre-existing vessels. Angiogenesis is essential for proper development and organ homeostasis, such as collateral formation, wound healing, and granulation. It is quite important to mention angiogenesis is not always healthy and could be associated with pathologic conditions such as DM; in this condiotion it would be considered as a pathologic angiogenesis. Angiogenesis results from the balanced functions of pro- and anti-angiogenic molecules.
Defects in the angiogenic balance may cause a shift towards either excessive or antiangiogenesis [18]. Abnormal angiogenesis were studied well in other body oragans in animal models [192021]. The abnormal angiogenesis that occurs in diabetic retinopathy has been well characterized in deficient (Ang2LacZ) mice models [19]. In diabetic retinopathy, the pericytes of the retinal capillaries are injured, which is associated with defective capillary function. Such capillary deficiency is associated with "in proper oxygen delivery and nutrient supply"; (which results in vascular endothelial growth factor (VEGF) and overproduction in the retina. This VEGF overproduction is also associated with abnormal angiogenesis and enhanced retinal capillary permeability, resulting in retinal dysfunction associated with loss of visual acuity in these patients [19]. Nyengaard and Rasch [20] identified abnormal glomerular capillaries in STZ-induced type 1 DM in rats. They determined that in diabetic animals, the total average surface area, length, and numbers of glomerular capillaries were elevated compared to the control groups. Similarly, db/db mice were found to show increased endothelial cell number and elongation of capillaries in their glomeruli [21]. These abnormal additional vessels which were found in diabetic animal, possess a thin wall at the basement membrane; while endothelial cells get swollen it presents that they are structurally immature and capable of causing increase in vascular permeability. An increase in capillary permeability often results in the extravasation of plasma protein as well as forming of lesions in diabetic nephropathy. Tahergorabi and Khazaei reported that quality of these blood vessels were not good enough in diabetic patients [26]. Consistency our findings showed that the blood vessel density increased in diabetic rats (DM+NS) testises. Furthermore, other findings in the current study showed, these rats did not only have a higher rate of apoptotic cells compared to healthy groups, but they also had significantly lower spermatogenesis score at the same time. Therefore, it seems the quality of the blood vessels are not sufficient enough in the current study as well. PTX-treated rats showed both decreased number of blood vessels and neovasculars notably were higher spermatogenesis score compared to diabetic rats. PTX reduces inflammatory parameters, as a pro-inflammatory cytokines and iNOS expression to indicate the potential benefit for the drug of choice in the treatment of DM and related pathologic conditions [23]. PTX conjointly has antioxidant properties. In consideration of PTX properties for anti-inflammatory and antioxidant effects, PTX could be a therapeutical alternative for the treatment of DM and the complications [23]. Accordong to Rasslan report, PTX inhibits microvascular construction [24]. On the other hand, Ersoy et al. proved in an animal model with ischemia-reperfusion, PTX adminstration decereased VEGF level [25]. In the current study of PTX adminstration through we found the following: the inhibition of microvascular costrction, decrease in VEGF level , and decrease in blood vessel density. The quality of improvement got much better.
According to the literature, DM negatively affects seminiferous tubal cells, the diameter, and spermatogenesis process during the developmental period [26]. DM caused significantly decreased in rats' testes, the numbers of Sertoli, and Leydig cells [14]. In insulin dependent DM, the function of Leydig cells, Sertoli cells, and production of testosterone decrease due to the lack of the stimulatory effect of insulin on those cells. In addition, an insulin-dependent DM decrease in FSH, which in turn, reduces LH levels, sperm production , and fertility [27282930].
Ricci et al. and Bal et al. have demonstrated that the tissues of testes, the cyto-architectures of seminiferous tubules, and the distribution of occludin change negatively in DM animals. The interstitial part of the tissues in testes were hypertrophic in several areas in these organs in the effected rats [3132]. The result of the current study have supported the findings and showed that increase in blood glucose, because DM caused quite abnormal changes in the function and histological parameters of the testes.
We observed that the treatment for DM by PTX improved the spermatogenesis process. The number of TUNEL-positive cells decreased following PTX treatment in DM rats. These findings matches the other studies [143334]. Najar et al. reported that PTX administration increased Sertoli cells and Leydig cells count in non-diabetic rates in comparison to the control groups. However, its effect in diabetic rats were significant [14]. Mc kinneyet et al. and Terriou reported that PTX improved testes function and the blood supplies; as a result, it caused a remarkable increase in the sperm motility in abnormal testes [3334].
In the study conducted by Cai et al., it was reported that blood supply and vascular circulation patterns improved by using endothelin antagonist treatment in DM experimental rates. [27]. In contrast, our results showed that vascular density in DM rats treated by 50 mg/kg/day PTX for 14 days decreased compared to the healthy control rats (NG). However, there was no significant change observed between DM+NS and the healthy control rats. Cai et al. also showed that the treatment of choice for diabetic animals by Bosentan as a endothelin antagonist prevented increase of vascular density and apoptosis in testes of diabetic rats [26]. Our results confirmaed Cai et al. study.
It was concluded that PTX administration to STZ-induced type 1 diabetic rats had a positive effect on blood vessel density and apoptotic cell numbers in diabetic rats; in addition, it improved spermatogenesis. More studies are recommended in order to carify molecular mechanisms of PTX administration on testicular tissue and to clarify clinical usefulness of this agent.
Acknowledgments
We express our appreciation to the Vice Chancellor of Research at Shahid Behesti University of Medical Sciences for financial support (grant no. 90-1-153-8183).
References
1. Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008; 4(1):46–54. PMID: 18220695.
2. Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, Reichner JS, Albina JE. Disruption of interleukin-1 signaling improves the quality of wound healing. Am J Pathol. 2009; 174(6):2129–2136. PMID: 19389930.

3. Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Textbook of complementary and alternative medicine. CRC Press;2003. p. 267.
4. Kolodny RC, Kahn CB, Goldstein HH, Barnett DM. Sexual dysfunction in diabetic men. Diabetes. 1974; 23(4):306–309. PMID: 4823910.

5. Fairburn C. The sexual problems of diabetic men. Br J Hosp Med. 1981; 25(5):484487489–491. PMID: 7025943.
6. Steger RW, Rabe MB. The effect of diabetes mellitus on endocrine and reproductive function. Proc Soc Exp Biol Med. 1997; 214(1):1–11. PMID: 9012356.

7. Mosher WD. Fecundity and infertility in the United States. Am J Public Health. 1988; 78(2):181–182. PMID: 3337335.

8. Aviado DM, Porter JM. Pentoxifylline: a new drug for the treatment of intermittent claudication. Mechanism of action, pharmacokinetics, clinical efficacy and adverse effects. Pharmacotherapy. 1984; 4(6):297–307. PMID: 6393073.

9. Rendell M, Bamisedun O. Skin blood flow and current perception in pentoxifylline-treated diabetic neuropathy. Angiology. 1992; 43(10):843–851. PMID: 1476272.

10. Aparicio NJ, Schwarzstein L, de Turner EA. Pentoxifylline (BL 191) by oral administration in the treatment of asthenozoospermia. Andrologia. 1980; 12(3):228–231. PMID: 7004272.

11. Babaei S, Bayat M, Nouruzian M, Bayat M. Pentoxifylline improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2013; 700(1-3):165–172. PMID: 23220163.

12. Soleimani Mehranjani M, Taefi R. The effects of sodium arsenite on the testis structure and sex hormones in vasectomised rats. Iran J Reprod Med. 2012; 10(6):571–580. PMID: 25246929.
13. Savaş C, Dindar H, Aras T, Yücesan S. Pentoxifylline improves blood flow to both testes in testicular torsion. Int Urol Nephrol. 2002; 33(1):81–85. PMID: 12090346.
14. Najar A, Piryae A, Babaei S, Bayat M. Effect of pentoxifylline on Sertoli and Leydig cells count of experimentally induced type 1 diabetes in male rats. Ann Mil Health Sci Res. 2013; 11(3):188–195.
15. Queiroz GCD, Oliveir VVG, Gueiros OG, Torres SM, Maia FCL, Tenorio BM, Morais RN, Silva JVA. Effect of pentoxifylline on the regeneration of rat testicular germ cells after heat shockd. Anim Reprod. 2013; 10(1):45–54.
16. Zhao Y, Tan Y, Dai J, Li B, Guo L, Cui J, Wang G, Shi X, Zhang X, Mellen N, Li W, Cai L. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011; 200(1-2):100–106. PMID: 21078376.

17. Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970; 1(1):2–25. PMID: 5527187.
18. Xu L, Kanasaki K, Kitada M, Koya D. Diabetic angiopathy and angiogenic defects. Fibrogenesis Tissue Repair. 2012; 5(1):13. PMID: 22853690.

19. Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008; 57(9):2495–2502. PMID: 18559662.
20. Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat. 2005; 207(6):813–821. PMID: 16367807.

21. Nyengaard JR, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia. 1993; 36(3):189–194. PMID: 8462766.

22. Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009; 58(7):1471–1478. PMID: 19564458.

23. Garcia FA, Rebouças JF, Balbino TQ, da Silva TG, de Carvalho-Júnior CH, Cerqueira GS, Brito GA, Viana GS. Pentoxifylline reduces the inflammatory process in diabetic rats: relationship with decreases of pro-inflammatory cytokines and inducible nitric oxide synthase. J Inflamm (Lond). 2015; 12:33. PMID: 25922592.

24. Rasslan R, Utiyama EM, Marques GM, Ferreira TC, da Costa VA, de Victo NC, Rasslan S, Montero EF. Inflammatory activity modulation by hypertonic saline and pentoxifylline in a rat model of strangulated closed loop small bowel obstruction. Int J Surg. 2014; 12(6):594–600. PMID: 24797690.

25. Ersoy YE, Ayan F, Himmetoglu S. Trace element levels in ischemia-reperfusion injury after left colonic anastomosis in rats and effects of papaverine and pentoxiphylline on vascular endothelial growth factor in anastomosis healing. Acta Gastroenterol Belg. 2011; 74(1):22–27. PMID: 21563650.
26. Tahergorabi Z, Khazaei M. Imbalance of angiogenesis in diabetic complications: the mechanisms. Int J Prev Med. 2012; 3(12):827–838. PMID: 23272281.

27. Cai L, Chen S, Evans T, Deng DX, Mukherjee K, Chakrabarti S. Apoptotic germ-cell death and testicular damage in experimental diabetes: prevention by endothelin antagonism. Urol Res. 2000; 28(5):342–347. PMID: 11127715.

28. Enzlin P, Mathieu C, Van Den Bruel A, Vanderschueren D, Demyttenaere K. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care. 2003; 26(2):409–414. PMID: 12547871.

29. Oksanen A. Testicular lesions of streptozotocin diabetic rats. Horm Res. 1975; 6(3):138–144. PMID: 130334.

30. Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004; 25(5):706–719. PMID: 15292100.

31. Ricci G, Catizone A, Esposito R, Pisanti FA, Vietri MT, Galdieri M. Diabetic rat testes: morphological and functional alterations. Andrologia. 2009; 41(6):361–368. PMID: 19891634.

32. Bal R, Türk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, Gür S, Nedzvetsky VS, Tykhomyrov AA, Andrievsky GV, Baydas G, Naziroglu M. rotective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocindiabetic male rats. Toxicology. 2011; 282(3):69–81. PMID: 21163323.
33. McKinney KA, Lewis SE, Thompson W. Persistent effects of pentoxifylline on human sperm motility, after drug removal, in normozoospermic and asthenozoospermic individuals. Andrologia. 1994; 26(4):235–240. PMID: 7978376.

34. Terriou P, Hans E, Giorgetti C, Spach JL, Salzmann J, Urrutia V, Roulier R. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000; 17(4):194–199. PMID: 10955242.
Figure 1
Photomicrograph of rats' testicular seminiferous tubules (H&E stain, 100×). In this section the blood vessels (arrows) were observed between transverse sections of seminiferous tubules. A: Normal glycemic (NG); B: Diabetes mellitus (DM) C: NG+normal saline (NS) D: DM+pentoxifylline (PTX).
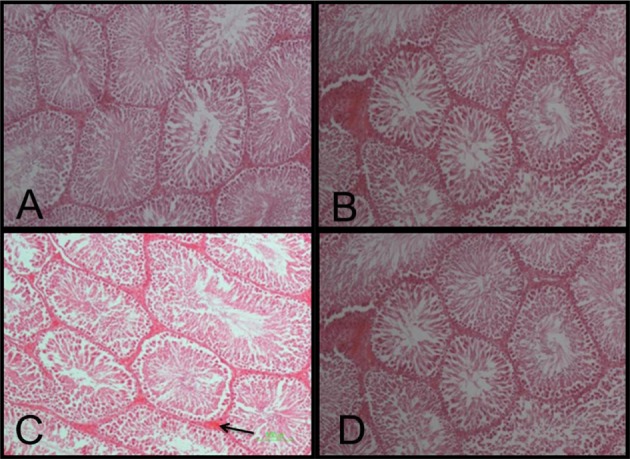
Figure 2
Changes in testicular blood vessel density in the studied groups: + indicates a significant difference between healthy controls and diabetic groups; control or normoglycemic group: NG, NG rats that received only normal saline (NS): NG+NS, Diabetics: DM+vehicle solution (NS), Experimental diabetic group: DM+pentoxifylline (PTX). Results were reported as mean±SEM.
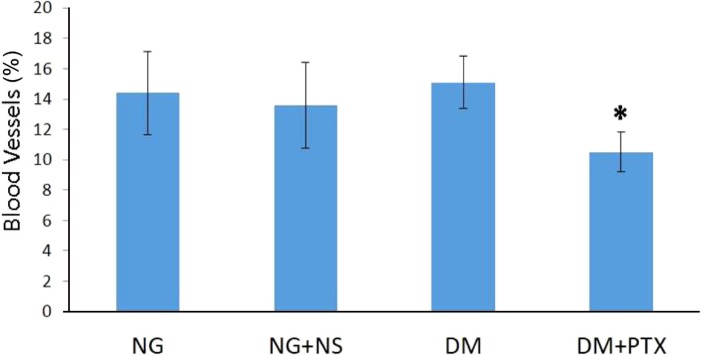
Figure 3
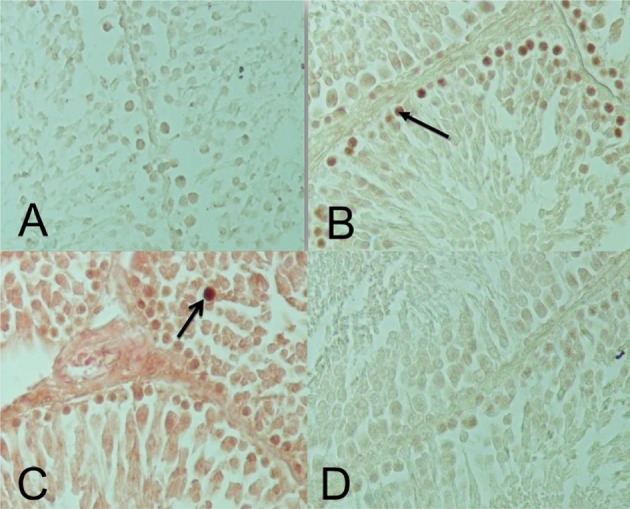
Photomicrograph of testicular seminiferous tubules from studied rats (TUNEL stain; 400×). In this method the cell nuclei (arrows) were observed as a dark brown color. A: NG group; B: DM+NS); C: NG+NS); D: DM+PTX.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download