Abstract
Although formaldehyde (FA) is known to be a major allergen responsible for allergic contact dermatitis, there are conflicting reports regarding correlation between FA exposure and interleukin (IL-4) expression. To investigate whether allergic responses including IL-4 expression were induced by repeated dermal exposure to low dose FA, alterations in the luciferase signal and allergic phenotypes were measured in IL-4/Luc/CNS-1 transgenic (Tg) mice containing luciferase cDNA under control of the IL-4 promoter after exposure to 4% FA for 2 weeks. High levels of luciferase were detected in the abdominal region of the whole body and submandibular lymph node (SLN) of FA treated mice. Additionally, the ear thickness and IgE concentration were significantly upregulated in the FA treated group when compared with the acetone olive oil (AOO) treated group. FA treated mice showed enhanced auricular lymph node (ALN) weight, epidermis and dermis thickness, and infiltration of inflammatory cells. Furthermore, the expression of IL-6 among T helper 2 cytokines was higher in the FA treated group than the AOO treated group, while vascular endothelial growth factor (VEGF) levels remained constant. Overall, the results presented herein provide additional evidence that various allergic responses may be successfully induced in IL-4/Luc/CNS-1 Tg mice after exposure to low dose FA for 2 weeks. The luciferase signal under the IL-4 promoter may reflect general indicators of the allergic response induced by exposure to low dose FA.
FA is widely used as a versatile chemical building block to produce FA-based resin and as an intermediate to produce other chemicals in industrial fields. Additionally, it is used in vaccines and disinfectants owing to its excellent antibacterial properties [1]. However, FA is a cause of allergic contact dermatitis and has the potential to induce occupational asthma [2,3].
Although various studies have reported the risk of FA to human health, there are conflicting reports regarding the relationship between FA exposure and IL-4 expression in various animals. Some of these reports have provided evidence that FA cannot stimulate an increase in IL-4 expression. High concentration FA (50%)-stimulated draining lymph node cells (LNC) showed no significant alteration in IL-4 secretion after topical exposure for 13 days, while 10% trimellitic anhydride (TMA) induced an increase in IL-4 secretion [4]. FA also did not induce IL-4 production by mouse bone marrow-derived mast cells after 72 h of culture [5]. Recent studies reported the opposite effects of FA on IL-4 expression; however, the concentrations of FA used differed among studies. The long-lasting expression of IL-4 mRNA was detected in draining lymph nodes and spleens of mice cutaneously exposed to 17.5% FA [6]. Moreover, exposure to 2, 5, and 10% FA induced remarkable increases in the expression of IL-4 mRNA in the cervical lymph node of BALB/c mice, while the expression of IL-4 mRNA in the ears of the same animals was only observed in response to 5 and 10% FA [7]. Furthermore, IL-4 production was induced by inhalation exposure to FA (3.6 mg/m3) for 3 days when compared to a vehicle treated group [8]. Therefore, we attempted to clarify these discrepancies in IL-4 response to FA exposure using IL-4/Luc/CNS-1 Tg mice containing luciferase cDNA under control of the human IL-4 promoter and enhancer of IL-4 (CNS-1).
In this study, we characterized the allergic responses induced by repeated dermal exposure to low dose FA using IL-4/Luc/CNS-1 Tg mice. The results confirmed the association of IL-4 during these responses. Specifically, the luciferase signal detected in our study showed that IL-4 may contribute to allergic responses after repeated dermal exposure to low dose FA for 2 weeks.
The animal protocols used in this study were reviewed and approved based on the ethical and scientific care procedures of the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2013-0385). The 8-week-old IL-4/Luc/CNS-1 Tg mice used in this study were kindly provided by the National Institute of Food and Drug Safety Evaluation of the Korea Food and Drug Administration (Osong, Korea), while HR1 mice were purchased from Central Lab Animal Inc. (Seoul, Korea). All mice were provided with ad libitum access to a standard irradiated chow diet (Samtako Inc., Korea) and water for 15 days. IL-4/Luc/CNS-1 Tg mice were handled in the Pusan National University-Laboratory Animal Resources Center accredited by the Korea Food and Drug Administration (FDA) (Accredited Unit Number-000231) and AAALAC International according to the National Institutes of Health guidelines (Accredited Unit Number-001525).
Eight-week-old IL-4/Luc/CNS-1 Tg mice (n=10) produced by mating of IL-4/Luc/CNS-1 Tg mice and HR1 mice randomly divided into two groups. In the first group (AOO treated group, n=5), 100 µL of AOO was repeatedly spread on the dorsum of the ears daily for 2 weeks. In the second group (FA treated group, n=5), 100 µL of 4% FA solution in vehicle (4:1 AOO, v/v: AOO) was repeatedly spread on the dorsum of the ears daily for 2 weeks. After final application, animals in the subset were subject to bioluminescence imaging analysis and further study.
Large numbers of IL-4/Luc/CNS-1 Tg mice were produced by mating IL-4/Luc/CNS-1 Tg mice and HR1 mice as described in a previous study [9]. Founder mice containing the IL-4/Luc/CNS-1 transgene were identified by PCR of tail-derived genomic DNA. For DNA-PCR, 10 pmol each of sense (5'-CTC GCA TGC CAG AGA TCC TA-3') and antisense (5'-CCA CAA CCT TCG CTT CAA AA-3') primers were added into the genomic DNA template mixture, after which the reaction mixtures were subjected to 25 cycles of amplification. Amplification was conducted in a thermal cycler (Perkin-Elmer, Waltham, MA, USA) under the following conditions: 30 sec at 94℃, 30 sec at 62℃, and 45 sec at 72℃. The amplified PCR products were then separated by 1% agarose gel electrophoresis, after which the band patterns were detected using a Kodak Electrophoresis Documentation and Analysis System 120 (Eastman Kodak, Rochester, NY, USA).
After final treatment, five immune organs including the ALN, thymus, spleen, SLN and mesenteric lymph node (MLN) were collected from scarified mice, and their weights were determined using an electronic balance (Mettler Toledo, Greifensee, Switzerland). Additionally, ear thickness was measured to determine the degree of allergic skin inflammation induced by FA treatment using a thickness gauge (Digimatic Indicator, Matusutoyo Co., Tokyo, Japan).
In vivo imaging was conducted using an IVIS imaging system (Xenogen, Oakland, CA, USA) as previously described [10]. Briefly, IL-4/Luc/CNS-1 Tg mice were anesthetized with Zoletil and injected i.p. with 150 mg/kg of D-luciferin (Sigma-Aldrich, MO, USA). At 10 minutes after D-luciferin injection, images of mice were taken for 3 min using an IVIS imaging system and photons emitted from specific regions were quantified using the Living Image software (Xenogen). The in vivo luciferase activity was then expressed in photons per second. The intensity of luminescence was analyzed using the Living Imaging software (Xenogen).
The serum IgE concentration was measured using an ELISA kit (Shibayagi, Inc., Gunma, Japan) according to the manufacturer's instructions. Briefly, wells coated with antibody were washed with washing solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0) three times, after which 50 µL of serum samples and standards diluted 20 fold with dilution solution were added to the wells and the plate was incubated for 2 h. Next, 50 µL of biotin-conjugated avidin was added after washing with the above solution and samples were incubated for 2 h. Horseradish peroxidase-conjugated detection antibodies were then diluted 5,000-fold with conjugate diluent (50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0) and transferred to each well. The plates were subsequently incubated at room temperature for 1 h, after which they were washed with washing solution three times. An enzyme reaction was subsequently initiated by adding substrate solution and incubating the plate at room temperature in the dark for 20 min. Finally, the reaction was terminated by adding 2M H2SO4 solution and the absorbance at 450 nm was measured.
Ear skins were removed from IL-4/Luc/CNS-1 Tg mice, fixed in 10% formalin, embedded in paraffin wax, routinely processed, and then sectioned into 5 µm thick slices. Next, the skin sections were stained with hematoxylin & eosin (H&E), after which they were examined by light microscopy at 100× and 400× magnification for the presence of edema and inflammatory cell accumulation and to determine the cellular morphology. The thickness of the epidermis and dermis and the number of lipid pores and inflammatory cells were also measured using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany).
Furthermore, the infiltration of mast cells into ear tissue was detected by staining with toluidine blue. After deparaffinization and dehydration, ear skin sections were stained with 0.25% Toluidine blue (Sigma-Aldrich) and examined by light microscopy for the presence of mast cells. The number of mast cells per specific area was measured with Leica Application Suite (Leica Microsystems) and viewed at 400× magnification.
RT-PCR was conducted to measure the relative quantities of luciferase mRNA in LN from IL-4/Luc/CNS-1 Tg mice. For RT-PCR analysis, LN were frozen in liquid nitrogen, then chopped with scissors and homogenized in RNAzol B solution (Tet-Test Inc., TX, USA). The isolated RNA was subsequently measured using a Biospec-nano spectrophotometer (Dong-il Shimadzu, Korea). Expression of the transgenes was assessed by RT-PCR using 5 µg of the total RNA from each tissue. Next, 500 ng of oligo-dT primer (Invitrogen, CA, USA) was annealed at 70℃ for 10 min. Complementary DNA, which was used as the template for further amplification, was synthesized by the addition of dATP, dCTP, dGTP, and dTTP with 200 units of reverse transcriptase. Subsequently, 10 pmol of the sense and antisense primers were added, and the reaction mixture was subjected to 32 cycles of amplification in a Perkin-Elmer Thermal Cycler as follows: 1 min at 94℃, 1 min at 62℃, and 1 min at 72℃. In each case, negative-RT controls were included to differentiate between DNA and RNA products. RT-PCR was also conducted using β-actin-specific primers to ensure RNA integrity. The primer sequences for mouse IL-4 were as follows: mIL-4, 5'-CAG TCG ATG TAC ACG TTC GTC AC-3' and 5'-CTC AGT ACT ACG AGT AAT CCA-3'. The sequences of the β-actin sense and antisense primers were 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3' and 5'-TAA AAC GCA GCT CAG TAA CAG TCC G-3', respectively. All samples were analyzed in triplicate, and the final PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
LN collected from a subset of the groups (n=5 per group) was homogenized using a PRO-PREP™ Solution Kit (iNtRON Biotechnology, Sungnam, Korea) supplemented with half of a protein inhibitor cocktail tablet (Roche, Penzberg, Germany). After homogenization, samples were centrifuged at 10,000×g for 10 min. The prepared proteins were then electrophoresed through a 10% SDS-PAGE gel, after which they were transferred to a nitrocellulose membrane (Amersham Biosciences, Corston, UK) for 2 h at 45 V in transfer buffer (25 mM Trizma-base, 192 mM glycine, and 20% methanol). Efficiency of the transfer as well as equal protein loading was evaluated by staining the membrane with Amido Black Staining Solution (Sigma-Aldrich) and the gel with Coomassie Blue. Appropriate dilutions of primary antibodies, anti-VEGF antibody (Pepro Tech., Rocky Hill, NJ, USA), anti-IL-6 (Santa Cruz Biotechnology, Dallas, TX, USA) and anti-β-actin (Sigma-Aldrich) were added to the membranes and allowed to hybridize overnight at 4℃. After the antibodies were removed, the membrane was washed three times in a solution composed of 10 mM Trizma-base (pH 7.6), 150 mM NaCl, and 0.05% Tween-20 for 10 min. The membrane was subsequently incubated with horseradish peroxidase-conjugated anti-secondary antibody for 1 h at room temperature, after which it was washed again as described above and developed using an enhanced chemiluminescence detection system (Amersham Bioscience). The results were quantified using the Image Analyzer System (Eastman Kodak 2000MM, NY, USA) and expressed as the fold-increase over control values.
To determine if repeated dermal exposure of low dose FA can induce the expression of IL-4, alterations in luciferase signals were measured throughout the body and in eight organs of IL-4/Luc/CNS-1 Tg mice using the Living Image software. Analysis of the whole body image revealed that the luciferase signal was only detected at high levels in the abdominal region of IL-4/Luc/CNS-1 Tg mice treated with FA, while it was not observed in the AOO treated group (Figure 1A). Moreover, analysis of the organ image revealed that higher luciferase signals only occurred in the SLN of IL-4/Luc/CNS-1 Tg mice treated with low dose FA relative to the AOO treated group (Figure 1B). Additionally, a significantly higher level of mouse IL-4 mRNA was detected in the SLN of mice treated with low dose FA (Figure 1C). Taken together, these results suggest that IL-4 expression was induced by repeated dermal exposure to low dose FA in the SLN of IL-4/Luc/CNS-1 Tg mice.
To characterize changes in ear phenotypes induced by repeated dermal exposure of low dose FA, the ear thickness and morphology were observed in IL-4/Luc/CNS-1 Tg mice over 2 weeks. The ear thickness rapidly increased in the FA treated group relative to the AOO treated groups (Figure 2C). Morphological analysis revealed that the outline of the ear vein became clear or thickened in the FA treated group relative to the AOO treated group, while ear color changed from a flesh tint to dark brown (Figure 2A, B). Therefore, these findings demonstrate that repeated dermal exposure to low dose FA could induce a severe increase in ear thickness and alteration of ear morphology.
To investigate whether low dose FA can induce an increase of immune-system functions, the weight of five organs associated with the immune system and serum IgE concentration were measured in IL-4/Luc/CNS-1 Tg mice. As shown in Table 1, exposure to low dose FA was found to induce an increase in ALN and thymus weight of IL-4/Luc/CNS-1 Tg mice, while the weight of the spleen, SLN and MLN were maintained. Furthermore, there was no difference in body weight between the AOO and FA treated groups.
Hyperproduction of IgE in blood serum is a characteristic of allergic hypersensitivity and an indicator of the magnitude of allergic immune response [11]. Therefore, the serum IgE concentration was measured in subset groups to determine if an increase in IgE concentration was induced by the repeated dermal exposure to low dose FA. A significant increase in serum IgE concentration was detected in FA treated IL-4/Luc/CNS-1 Tg mice relative to AOO treated mice (Figure 2D). Therefore, these results suggest that the exposure to low dose FA may contribute to enhancement of the ALN weight, thymus weight and IgE concentration in IL-4/Luc/CNS-1 Tg mice.
Changes in ear tissue histology in response to low dose FA were measured in the ear tissue of IL-4/Luc/CNS-1 Tg mice after exposure to low dose FA. The epidermis and dermis was thicker in the FA treated group than the AOO treated group, while lichenification was widely observed in the epidermal surface. Moreover, FA treatment resulted in a nearly 2-fold increase in adipocyte and inflammatory cells in the dermis layer relative to the AOO treated group (Figure 3A, B). Furthermore, it was well known that mast cells play an important role in asthma, eczema, itch, allergic rhinitis and allergic conjunctivitis [12]. Therefore, infiltration of mast cells in ear skin sections of FA treated IL-4/Luc/CNS-1 Tg mice were observed after staining with toluidine blue. The number of infiltrated mast cells was higher in the dermis of the FA treated group than the AOO treated group (Figure 3Ae, f, Be). Taken together, these results indicate that the exposure of low dose FA could induce histological changes in AD morphology, including an increase in the epidermis and dermis, enhancement of adipocytes and infiltration of inflammatory cells.
In addition, some of the chemokines and cytokines secreted from irritant sites could contribute to the regulatory mechanism responsible for differences in skin irritation following exposure to various concentration of PA [9,13]. Therefore, to determine if exposure to low dose FA was accompanied by the secretion of cytokines, the expression of IL-6 and VEGF was measured in ear tissue of IL-4/Luc/CNS-1 Tg mice. The expression pattern of the two cytokines differed greatly between groups. Specifically, the expression of IL-6 was about 2 times higher in the low dose FA treated group than the AOO treated group, while VEGF expression was maintained a constant level (Figure 4). Therefore, these results showed that exposure to low dose FA may stimulate increased IL-6 expression in the ear tissue of IL-4/Luc/CNS-1 Tg mice.
FA sensitizes the skin and induces allergic skin inflammation in humans through regulation of the secretion of T helper 1 and 2 cytokines [6,7]. However, several studies have shown conflicting results regarding the correlation between FA exposure and IL-4 expression. Therefore, we characterized the allergic response induced by repeated dermal exposure to low dose FA using IL-4/Luc/CNS-1 Tg mice, as well as the association of IL-4 with these responses. The results presented herein provide evidence that exposure to low dose FA may induce an allergic response characterized by increased IL-4 secretion, ear swelling, epidermis and dermis thickness, mast cell infiltration and IL-6 expression.
Most studies conducted to date have applied high concentrations of FA to investigate the effects of FA on IL-4 expression. BALB/c strain mice topically exposed to 50% FA showed no significant change in IL-4 expression, while IFN-γ rapidly increased with time [4]. However, IL-4 expression was increased in the draining lymph node and ear skin of mice following repeated cutaneous exposure to 17.5% FA [6], as well as in the cervical lymph node and ear of mice exposed to 5-10% FA [7]. The expression of IL-4 mRNA was not induced in the cervical lymph node and ear of mice exposed to 2% FA [7]. In this study, 4% FA solution was applied to the ear skin of IL-4/Luc/CNS-1 Tg mice to investigate the correlation between exposure to low dose FA and IL-4 response. The luciferase signal under the control of human IL-4 promoter and mouse IL-4 mRNA expression was dramatically enhanced by epidermal exposure to 4% FA, indicating that this level of FA solution may be the minimum concentration required to induce IL-4 response.
A previous investigation showed that treatment with 2, 5 and 10% FA for 5 weeks induced remarkable ear swelling, although the magnitude of the peak response was enhanced proportionally in response to increasing FA dose and the number of exposures [7]. Similar results were observed in our study; however, ear swelling was fully induced by application to the dorsal and ventral surfaces of both ear lobes three times a week for 2 weeks. Furthermore, the invasion of inflammatory cells including neutrophils, eosinophils and monocytes, as well as hypertrophy of the epidermis, was detected in mice exposed to 5 and 10% FA [7]. In this study, IL-4/Luc/CNS-1 Tg mice exposed to 4% FA for 2 weeks showed similar changes in H&E and toluidine blue stained ear sections.
IL-6 is believed to play an important role in ongoing chronic skin irritation as well as to induce the infiltration of mononuclear cells [14,15]. VEGF is also known as a potent mediator of angiogenesis that stimulates the proliferation and migration of endothelial cells, increases vascular permeability and induces the expression of some adhesion molecules on endothelial cells [16,17]. Furthermore, these two cytokines were found to be significantly upregulated in C57BL/6 mice following treatment with phthalic anhydride (PA) [9]. In this study, we tested the exposure effects of low dose FA on IL-6 and VEGF expression. As shown Figure 4, the expression of IL-6 was enhanced in the ear tissue of IL-4/Luc/CNS-1 Tg mice following repeated exposure to low dose FA, providing additional evidence that IL-6 is a major cause of irritant dermatitis in response to FA in mice.
In this study, allergic responses including IL-4 expression induced by repeated dermal exposure to low dose FA in IL-4/Luc/CNS-1 Tg mice were characterized based on the luciferase signal and general AD phenotypes after exposure for 2 weeks. The results showed that exposure to low dose FA may induce an increase in the luciferase signal, lymph node weight, thickness of the epidermis and dermis, inflammatory cell infiltration and IL-6 expression.
Acknowledgments
We thank Jin Hyang Hwang, the animal technician, for directing the Animal Facility and Care at the Laboratory Animal Resources Center. This work was supported for two years by a Pusan National University Research Grant.
References
1. Günther R, Walter D, Armin OG, Albrecht H. Formaldehyde. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH;2002.
2. Marzulli FN, Maibach HI. The use of graded concentrations in studying skin sensitizers: experimental contact sensitization in man. Food Cosmet Toxicol. 1974; 12(2):219–227. PMID: 4459237.

3. Nethercott JR, Holness DL. Contact dermatitis in funeral service workers. Contact Dermatitis. 1988; 18(5):263–267. PMID: 2970932.

4. Dearman RJ, Basketter DA, Evans P, Kimber I. Comparison of cytokine secretion profiles provoked in mice by glutaraldehyde and formaldehyde. Clin Exp Allergy. 1999; 29(1):124–132. PMID: 10051711.

5. Saneyoshi K, Nohara O, Imai T, Shiraishi F, Moriyama H, Fujimaki H. IL-4 and IL-6 production of bone marrow-derived mast cells is enhanced by treatment with environmental pollutants. Int Arch Allergy Immunol. 1997; 114(3):237–245. PMID: 9363904.

6. Xu B, Aoyama K, Takeuchi M, Matsushita T, Takeuchi T. Expression of cytokine mRNAs in mice cutaneously exposed to formaldehyde. Immunol Lett. 2002; 84(1):49–55. PMID: 12161283.

7. Saito A, Tanaka H, Usuda H, Shibata T, Higashi S, Yamashita H, Inagaki N, Nagai H. Characterization of skin inflammation induced by repeated exposure of toluene, xylene, and formaldehyde in mice. Environ Toxicol. 2011; 26(3):224–232. PMID: 19904815.

8. De Jong WH, Arts JH, De Klerk A, Schijf MA, Ezendam J, Kuper CF, Van Loveren H. Contact and respiratory sensitizers can be identified by cytokine profiles following inhalation exposure. Toxicology. 2009; 261(3):103–111. PMID: 19422874.

9. Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR, Lee JW, Lee CK, Hwang DY. IL-6, VEGF, KC and RANTES are a major cause of a high irritant dermatitis to phthalic anhydride in C57BL/6 inbred mice. Allergol Int. 2010; 59(4):389–397. PMID: 20864798.

10. Lee YJ, Kim JE, Kwak MH, Go J, Kim DS, Son HJ, Hwang DY. Quantitative evaluation of the therapeutic effect of fermented soybean products containing a high concentration of GABA on phthalic anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. Int J Mol Med. 2014; 33(5):1185–1194. PMID: 24604257.

11. Gao XK, Nakamura N, Fuseda K, Tanaka H, Inagaki N, Nagai H. Establishment of allergic dermatitis in NC/Nga mice as a model for severe atopic dermatitis. Biol Pharm Bull. 2004; 27(9):1376–1381. PMID: 15340222.

12. Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003; 15(4):399–404. PMID: 12891053.

13. Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006; 212:238–255. PMID: 16903918.

14. Kapp A. The role of eosinophils in the pathogenesis of atopic dermatitis--eosinophil granule proteins as markers of disease activity. Allergy. 1993; 48(1):1–5. PMID: 8457021.
15. Dubois GR, Bruijnzeel PL. IL-4-induced migration of eosinophils in allergic inflammation. Ann N Y Acad Sci. 1994; 725:268–273. PMID: 8030998.

16. Detmar M, Brown LF, Schön MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998; 111(1):1–6. PMID: 9665379.

17. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004; 56(4):549–580. PMID: 15602010.

Figure 1
Detection of luciferase signal in the whole body (A) and each organ (B) of IL-4/Luc/CNS-1 Tg mice. Mice were treated with AOO and FA for 2 weeks and then imaged at 24 h after final treatment using the LivingImage software. The color overlay on the image represents the photons per second emitted from the organs in accordance with the pseudocolor scale shown next to the image. In this image, red indicates the highest number of photons per second, while blue indicates the lowest. L, lung; K, kidney; S, spleen; H, heart; SLN, submandibular lymph node; MLN, mesenteric lymph node; T, thymus; P, pancreas. (C) The expression of mouse IL-4 mRNA in SLN was measured by RT-PCR using a specific primer. Data shown are the means±SD (n=5). a, P<0.05 compared to the AOO treated group.
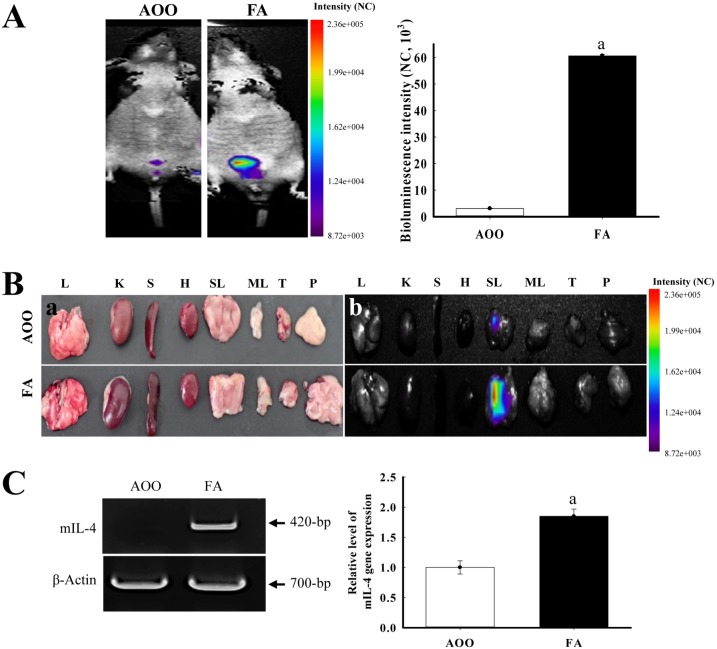
Figure 2
Alteration of ear phenotypes and IgE concentration. After repeated application of FA solution (4%) to the ear of IL-4/Luc/CNS-1 Tg mice for 2 weeks, the outline of the ear vein in two groups was analyzed using the picture image (A and B), and ear thickness of the mice was measured with a thickness gauge on each experimental day (C). Arrows indicate the vein in the ear. The serum used to measure the IgE concentration was prepared from blood samples collected from the abdominal vein of the mice. The IgE concentration in serum was quantified by an enzyme-linked immunosorbent assay (D). Data shown are the means±SD (n=5). a, P<0.05 compared to the AOO treated group.
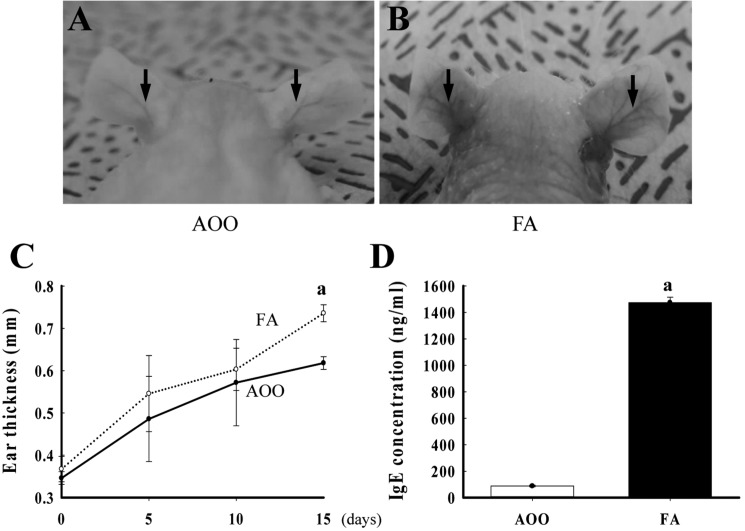
Figure 3
Histological alteration of ear skin. FA solution (4%) was repeatedly applied to the dorsum of the ear of IL-4/Luc/CNS-1 Tg mice. After 2 weeks, ear tissues were collected from AOO or FA treated IL-4/Luc/CNS-1 Tg mice. The histological changes were determined as described in the Materials and Methods. The slide sections of ear tissue were stained with hematoxylin & eosin and then observed at 100× or 400× magnification (Aa-d and Ba-d). Slide sections of ear tissue were stained with toluidine blue and then observed at 400× magnification (Ae and f, and Bc). Arrow heads indicate the infiltrated mast cells in the dermis of the ear. Data shown are the means±SD (n=5). a, P<0.05 compared to the AOO treated group.
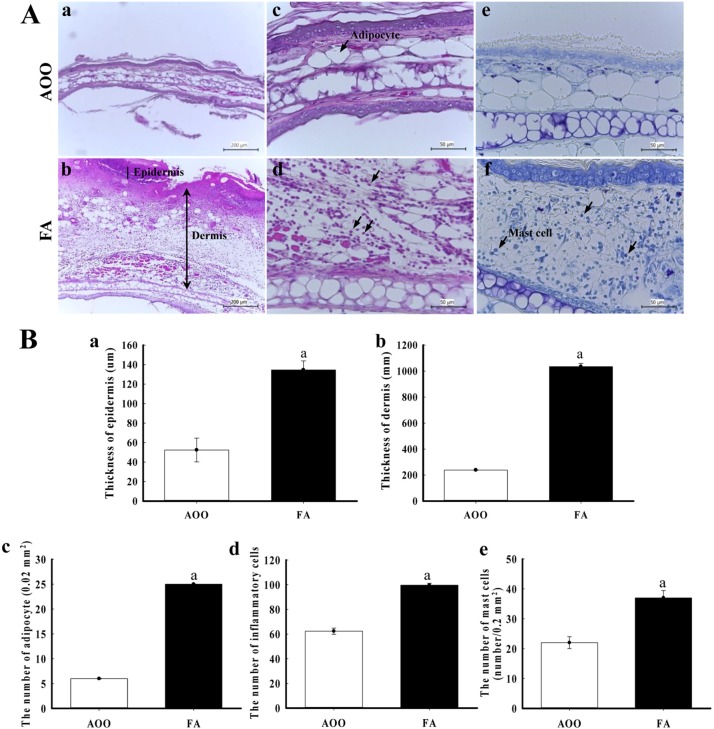
Figure 4
IL-6 and VEGF expression in ear tissue. After detection of specific bands with two primary antibodies, the intensity of each band was determined using an imaging densitometer and the relative level of each protein was calculated based on the intensity of actin protein as an endogenous control. Data represent the mean±SD from three replicates. a, P<0.05 compared to the AOO-treated group.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download