Abstract
This study was performed to investigate the expression of two porcine endogenous retrovirus (PERV) elements, PERV gag and full-length conserved PERV, in blood cells collected periodically from organ-recipient monkeys that underwent pig to non-human primate xenotransplantation. The heart and kidney-respectively acquired from α-1,3-galactosyltransferase knockout (GT-KO) pigs that survived for24 and 25 days-were xenografted into cynomolgus monkeys. The two PERV elements expressed in the xenografted GT-KO pig organs were not present in the blood cells of the recipient monkeys. In the present study, we deduced that PERVs are not transmitted during GT-KO pig to monkey xenotransplantation.
Organ transplantation is dependent on the availability of implantation organs, and a major limitation is the serious imbalance between the number of organ donors and that of the recipients. To bridge the gap, pig-to-human xenotransplantation has been considered a potential alternative to allotransplants [1]. In spite of advances in success of xenotransplantation, subjects may be exposed to porcine cells in vivo for longer periods, thereby increasing the risk of porcine endogenous retrovirus (PERV) transmission, which is a potential public health risk [10]. Several subclasses of infectious PERVs have been described according to pig types such as NIH-minipig [11]. Among the functional subtype genes of PERVs, the gene gag is highly conserved and its expression is essential for virion production [9]. The expression of gag in blood cells of xeno-organ recipients could imply an infection of PERV particles originating from pig tissues. Furthermore, the investigation of synthetic full-length conserved PERVs is essential to evaluate the risks associated with xenotransplantation. The expression of the two PERV elements may offer suggestive evidence for PERV transmission and infection during xenotransplantation. Therefore, we conducted to investigate the expression of two PERV elements: 1) the PERV gag and 2) the full-length conserved PERV in blood cells periodically collected from recipient cynomolgus monkeys after pig-to-monkey xenotransplantation.
The study protocol and standard operating procedures were reviewed and approved by the Orient Genia Institutional Animal Care and Use Committee (IACUC No. ORIENT-IACUC-11104) for monkeys, and by the National Institute of Animal Science's Institutional Animal Care and Use Committee (No. 2012-D-008) for GT-KO pigs. Cynomolgus monkeys (Macaca fascicularis; China, 4 to 5 kg; blood type A; n=2; Orient Genia, Inc., Seongnam, Republic of Korea) were used as organ recipients. Homozygous GT-KO pigs (n=2; blood type A; 5 to 7 kg; National Institute of Animal Science, RDA, Suwon, Republic of Korea) were housed in a specific pathogen-free facility to be used as organ donors [3]. Surgical procedures and immunization protocols were performed as previously described [6]. Briefly, the donor heart and kidney were transplanted into the abdomen of the recipients by anastomosis of the donor aorta to the recipient aorta and by the donor pulmonary artery to the recipient inferior vena cava (IVC) in heart transplantation and donor renal artery to recipient aorta, and donor renal vein to recipient IVC, respectively. Recipients received induction therapy with rabbit anti-thymocyte globulin 5 mg/kg/day (Genzyme, Cambridge, USA) for 4 times, rituximab 10 mg/kg/day (Roche, Basel, Switzerland) for 2 times, and Cobra venom factor 0.05mg/kg/day (Quidel, San Diego, USA)for5 times. Whole blood from the recipient monkeys was collected in K2 EDTA tube (BD Vacutainer, Franklin Lakes, USA). Total RNA from the blood cells was extracted using Trizol reagent (Invitrogen, Carlsbad, USA). Primer sets were designed to amplify GAPDH (For: 5'-ggaatcccatcaccatcttccagg-3'; Rev: 5'-gagccccagccttctccatg-3') (NM_002046), PERV gag (For: 5'-tcaggcggtacaccccttt-3'; Rev: 5'-gatcacgtaactcagc ctcctgtaa-3') [2], and full-length conserved PERV (For: 5'-tccagggctcataatttgtc-3'; Rev: 5'-tgatggccatccaacatcga-3') [7], respectively.
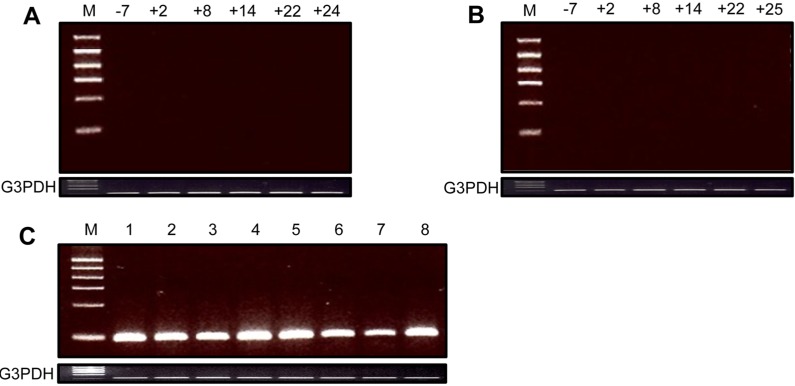
The PERV gag element was not detected during the survival periods in the blood cells of recipient cynomolgus monkeys transplanted with GT-KO pig heart and kidney (Figure 1A and 1B). In contrast, it was strongly expressed in biopsied and autopsied heart and kidney xenografts from GT-KO pigs (Figure 1C). The full-length conserved PERV was also not found during the survival periods in the blood cells of the recipient monkeys (Figure 2A and 2B), although it was expressed in the xenografted GT-KO pig organs (Figure 2C).
This is the first report of a PERV transmission after pig to monkey xenotransplantation in Korea. Based on the xenotransplantation experiments, we obtained several hematological results: 1) there was no evidence of hyperacute immune rejection during the survival periods; 2) no characteristic disseminated intravascular coagulation and thrombocytopenia, and 3) stable levels of troponin I, reflecting that the function of the grafts was normal after transplantation. And both the PERV gag and full-length conserved PERV elements were not detected in the blood cells of the recipient monkeys after heart and kidney xenotransplantation, respectively. PERVs have been shown to infect human cells in vitro, and have been found to be actively transcribed, translated, and infectious in other species in vivo after the transplantation of pig tissues [5,12]. However, several researchers reported that the patients, who were screened after receiving porcine tissue, were found to be negative for PERV transmission [4,8].Thus far, there are some controversies on the risk of PERV transmission or infection following xenotransplantation. However, ongoing research on several subtypes and their characteristics is of prime importance to rule out any associated risks caused by PERVs. Although we could not find any clues on the transmission of PERVs in blood cells of recipient monkeys, it is difficult to conclude that no PERV transmission occurs during pig to monkey xenotransplantation because of short-term adaptation. Therefore, further studies are required for long term monitoring of PERV expression in recipients.
Acknowledgment
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ008331)" Rural Development Administration, Korea.
References
1. van der Windt DJ, Bottino R, Kumar G, Wijkstrom M, Hara H, Ezzelarab M, Ekser B, Phelps C, Murase N, Casu A, Ayares D, Lakkis FG, Trucco M, Cooper DK. Clinical islet xenotransplantation: how close are we? Diabetes. 2012; 61(12):3046–3055. PMID: 23172951.
2. Dieckhoff B, Petersen B, Kues WA, Kurth R, Niemann H, Denner J. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation. 2008; 15(1):36–45. PMID: 18333912.

3. Hwang S, Oh KB, Kim DH, Woo JS, Shim H, Yun IJ, Park JK, Im GS. Production of α1,3-galactosyltransferase (GalT) double knock-out (-/-) transgenic pigs for xenotransplantation. Korean J Embryo Transf. 2012; 27(1):9–14.
4. Irgang M, Sauer IM, Karlas A, Zeilinger K, Gerlach JC, Kurth R, Neuhaus P, Denner J. Porcine endogenous retroviruses: no infection in patients treated with a bioreactor based on porcine liver cells. J Clin Virol. 2003; 28(2):141–154. PMID: 12957184.

5. Karlas A, Irgang M, Votteler J, Specke V, Ozel M, Kurth R, Denner J. Characterisation of a human cell-adapted porcine endogenous retrovirus PERV-A/C. Ann Transplant. 2010; 15(2):45–54. PMID: 20657519.
6. Kim H, Chee HK, Yang J, Hwang S, Han KH, Kang J, Park JH, Kim JS, Lee SJ, Ock SA, Park MH, Park KS, Byeongchun L, Cho K, Noh J, Park W, Yun IJ, Ahn C. Outcomes of alpha 1,3-GT-knockout porcine heart transplants into a preclinical nonhuman primate model. Transplant Proc. 2013; 45(8):3085–3091. PMID: 24157041.

7. Moon HJ, Kim HK, Park SJ, Lee CS, Song DS, Kang BK, Park BK. Comparison of the age-related porcine endogenous retrovirus (PERV)expression using duplex RT-PCR. J Vet Sci. 2009; 10(4):317–322. PMID: 19934597.
8. Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, Otto E. The XEN 111 Study Group. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999; 285(5431):1236–1241. PMID: 10455044.

9. Quinn G, Wood J, Suling K, Arn S, Sachs DH, Schuurman HJ, Patience C. Genotyping of porcine endogenous retroviruses from a family of miniature swine. J Virol. 2004; 78(1):314–319. PMID: 14671113.

10. Ritzhaupt A, Van Der Laan LJ, Salomon DR, Wilson CA. Porcine endogenous retrovirus infects but does not replicate in nonhuman primate primary cells and cell lines. J Virol. 2002; 76(22):11312–11320. PMID: 12388691.

11. Wilson CA. Porcine endogenous retroviruses and xenotransplantation. Cell Mol Life Sci. 2008; 65(21):3399–3412. PMID: 18818871.
12. Wood JC, Quinn G, Suling KM, Oldmixon BA, Van Tine BA, Cina R, Arn S, Huang CA, Scobie L, Onions DE, Sachs DH, Schuurman HJ, Fishman JA, Patience C. Identification of exogenous forms of human-tropic porcine endogenous retrovirus in miniature Swine. J Virol. 2004; 78(5):2494–2501. PMID: 14963150.

Figure 1
Expression of PERV gag element in the blood cells from the recipient monkeys. A: Heart xenografted monkey: days before (-) and after (+) xenotransplantation; B: Kidney xenografted monkey: days before (-) and after (+) xenotransplantation; C: Xenografted GT-KO pig organs; 1: kidney-pre-xenotransplantation; 2: kidney-biopsy (day+9); 3: kidney-autopsy (day+25) connected with blood vessel; 4: kidney-autopsy (day+25) main body; 5: heart-autopsy (day+24) lower part; 6: heart-autopsy (day+24) middle part; 7: heart-autopsy (day+24) upper part; 8: heart-autopsy (day+24) connected with blood vessel. The PCR conditions were as follows: initial heating at 94℃ for 5 min; 35 cycles of 94℃ for 40 s, 55℃ (GAPDH) and 59℃ (PERV-gag) for 30 s and 72℃ for 40 s; and a final elongation step for 10 min at 72℃. The size of PCR products was 120 bp (GAPDH) and 150 bp (PERV-gag), respectively.
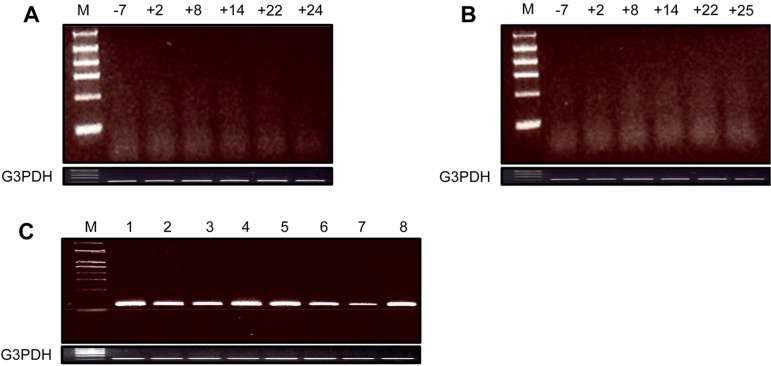
Figure 2
Expression of full-length conserved PERV in the blood cells from the recipient monkeys. A: Heart xenografted monkey: days before (-) and after (+) xenotransplantation; B: Kidney xenografted monkey: days before (-) and after (+) xenotransplantation; C: Xenografted GT-KO pig organs; 1: kidney-pre-xenotransplantation; 2: kidney-biopsy (day+9); 3: kidney-autopsy (day+25) connected with blood vessel; 4: kidney-autopsy (day+25) main body; 5: heart-autopsy (day+24) lower part; 6: heart-autopsy (day+24) middle part; 7: heart-autopsy (day+24) upper part; 8: heart-autopsy (day+24) connected with blood vessel. The PCR conditions were as follows: initial heating at 94℃ for 5 min; 35 cycles of 94℃ for 40 s, 55℃ (for GAPDH and full-length conserved PERV) for 30 s and 72℃ for 40 s; and a final elongation step for 10 min at 72℃. The size of PCR products was 120 bp (GAPDH) and 108 bp (full-length conserved PERV), respectively.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download