Abstract
According to WHO global estimates from 2008, more than 1.4 billion adults were overweight and among them, over 200 million men and 300 million women were obese. Although the main treatment modalities for overweight and obese individuals remain dieting and physical exercise, the synthetic anti-obesity medications have been increasingly used due to their perceived convenience. Generally, anti-obesity medications are classified as appetite suppressants or fat absorption blockers. In the present study, we examined the adverse side-effects in respect of behavior changes of phentermine and Ephedra sinica (mahuang) that are anti-obesity drugs currently distributed to domestic consumers. Phentermine is mainly classified as an anorexing agent and mahuang a thermogenic agent. Because phentermine and mahuang are considered to display effectiveness through the regulation of nerve system, their potential influences of on behavioral changes were examined employing animal experiments. From the results of experiments testing locomotor activity through the use of treadmill, rota-rod, and open field system, phentermine and mahuang were commonly revealed to induce behavioral changes of rats by reducing a motor ability, an ability to cope with an external stimulus, and a sense of balance or by augmenting wariness or excitement. These adverse effects of phenternime and mahuang in behavioral changes need to be identified in humans and anti-obesity medications such as phentermine and mahuang should be prescribed for only obesity where it is anticipated that the benefits of the treatment outweigh their potential risks.
Overweight or obesity is generally defined as having more body fat than is optimally needed. Body fat is a necessary component for the proper functioning of hormonal, reproductive, and immune systems. It is also used for thermal insulation, shock absorption for sensitive areas, and energy production [1]. But the accumulation of too much storage fat can cause overweight or obesity, leading to the impairment in movement and flexibility, the alteration in body appearance, and further the occurrence of related diseases including cardiac disorders and type 2 diabetes mellitus [2,3].
On the basis of body mass index (BMI), overweight is usually described as a BMI between 25 and 30 and obesity as a BMI of 30 or more. According to WHO global estimates from 2008, more than 1.4 billion adults were overweight and among them, over 200 million men and 300 million women were obese. WHO is also warning that overweight and obesity are the fifth leading risk for global death (http://www.who.int/mediacentre/factsheets/fs311/en). Treatments for overweight or obesity are known to be a proper diet and tailored exercise program [4,5], and sometimes some medications developed as anti-obesity purpose by functioning as appetite suppressants or fat absorption blockers [6,7].
Although the main treatment modalities for overweight and obese individuals remain dieting and physical exercise, the synthetic anti-obesity medications have been increasingly used due to their perceived convenience. For examples of anti-obesity medications, orlistat, an intestinal fat absorption inhibitor, is currently approved by the FDA for long term use in spite of some side-effects such as oily bowel movements [8,9,10]. Rimonabant is a recently developed anti-obesity medication that acts centrally on the brain thus decreasing appetite and peripherally by increasing thermogenesis and energy expenditure [11]. However, due to safety concerns, primarily psychiatric in nature, rimonabant has not received approval in the U.S. or Canada. Lorcaserin is a weight-loss drug that has serotonergic properties and acts as an appetite suppressant [12,13]. The application of lorcaserin was also recently retracted by the FDA based on concerns over both safety and efficacy including headache, upper respiratory tract infection, nausea, and depression ("FDA Issues Complete Response Letter for Lorcaserin New Drug Application" 23 October 2010). Therefore, because of the potential side effects, anti-obesity medications are recommended to be prescribed for only obesity where it is anticipated that the benefits of the treatment outweigh its risks [6,14].
In the present study, we examined the adverse side effects in respect of behavior changes of phentermine and Ephedra sinica (mahuang) that are anti-obesity drugs currently distributed to domestic consumers. Phentermine is one of appetite-suppressant medications, which was approved for short-term use in the treatment of obesity by the U.S. FDA in 1959, and currently remains available [15]. Mahuang is an oriental medicinal herb traditionally used to treat shivering fits and asthma and has been also prescribed to treat obesity [16,17]. Anti-obesity effect of mahuang is attributed to ephendrin, a major alkaloid found in mahuang, which is classified as a thermogenic agent having a stimulation function on sympathetic nerve [18,19]. As the anti-obesity effects of phentermine and mahuang are mainly associated with the regulation of nerve system, we investigated whether they may influence behavioral changes of experimental animals to forecast the behavioral disturbances in humans as a result of the side effects of these medications.
Phentermine (Dietamin®, Daewoong pharm., Seoul, Korea) was used by solubilizing in 1× phosphate-buffered saline (1× PBS; pH 7.4). Mahuang was brewed and concentrated via evaporation. The extract of mahuang was used by solubilizing in 1× PBS. PBS was used as a vehicle of drugs and a negative control.
Eight-week-old male Sprague-Dawley rats (n=10) weighing 250 g on average were purchased from a commercial breeder (Daehan Biolink, Eumseong, Korea). They were housed in an environmentally controlled room with constant temperature (23±3℃), relative humidity (50±10%), and 12-h light cycle. Rats were fed a standard commercial rodent chow (Daehan Biolink). Three rats were allocated to each group. The rats of phentermine group were orally administered with 0.0058 g phentermine in 2 mL of PBS. The rats of mahuang group were also orally administered with 1.0 g mahuang in 2 mL of PBS. The rats of control group were orally administered with only 2 mL of PBS. The dose of each drug was determined 10 fold higher on the basis of an actual dosage applied to humans. Behavioral tests were conducted 30 minutes after oral administration of each drug. All animal experiments were in accordance with the Standard Operation Procedures of Laboratory Animal and approved by Institutional Animal Care and Use Committee of Laboratory Animal Research Center, Chungbuk National University.
Treadmill system has a rolling belt with adjustable speed and slope enabling forced exercise training and accurate testing of physical activity in rodents [20]. To encourage the rats to continue running, it also has a metal bar that provides a foot shock (0.4 mA micro current) that causes a tingling sensation. If rodents have a normal nervous system, they learn to continue running to avoid this shock. Generally, the moving distance and staying time on a metal bar are measured during three-times of foot shock. First, for testing the capacity for locomotion and ambulatory function, rats were subjected to a running test at 15 cm/sec on a treadmill (Coulbourn Instruments, North America) and the moving distance was measured for each rat. Next, the staying time which is the elapsed time for a rat to return to the front of a moving platform when a foot shock stimulation was applied was measured for testing the ability to cope with an external stimulus and a sense of direction.
For examining a sense of motor coordination and balance, rats were subjected to a moving test on rota-rod test system (Panlab Technology, Barcelona, Spain) of which rotation speed was accelerated from 5 rpm to 40 rpm within 30 sec in an early stage and maintained at 40 rpm afterwards. The time of falling off the rod was recorded for each rat according to a previous study [21].
Spontaneous activities and exploratory behaviors of rats were evaluated using a video tracking system (Smart v2.5; Panlab, Barcelona, Spain) connected to a CCTV monitor (Samsung, Changwon, Korea). Rats were placed in a quiet black chamber (60×60 cm2 chamber, 40-cm-high walls, with its floor divided into 25 equal squares) with dim light, and the time spent for each type of movements, i.e., resting (below 100 cm/sec), slow-moving (100~300 cm/sec), and fast-moving (300~500 cm/sec), was recorded for 5 min for each mouse according to a previous study [21]. Time interval between movements was 5 seconds.
Data are presented as mean±SEM. The statistical significance between group comparisons for behavioral data was determined by 1-way analysis of variance (ANOVA), followed by post-hoc Dunnett's multiple comparison test. A value of P<0.05 was considered to be statistically significant.
In a locomotor activity test using a treadmill, the moving distance of the rats administered with phentermine, or mahuang was revealed to be remarkably shorter than that of the rats fed PBS as a negative control (Figure 1). The mice of control group moved as long as 78 m for 15 min on average, but the mice fed phentermin, or mahuang moved only 2 m, or 12 m, respectively. Therefore, it can be said that the capacity for locomotion and ambulatory function of the rats fed these drugs was significantly decreased compared with that of control rats. On the other hand, the staying time of the rats administered with drugs when a foot shock was applied was shown to be longer than that of the rats fed PBS as shown in Figure 2. Especially, the staying time of the rats fed mahuang was above 4 sec, while that of rats of control group was about 0.5 sec, which means that the ability to cope with an external stimulus and a sense of direction of the rats administered with drugs were slowed down about 8 times compared with those of control rats.
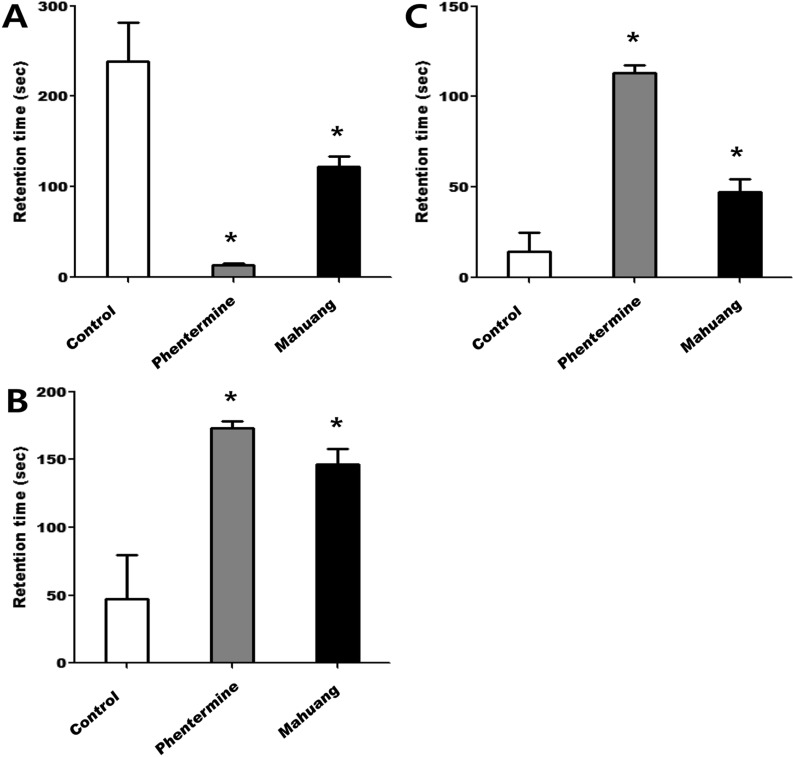
Next, in a test examining a sense of balance using a rota-rod, the moving time on a rotating rod of the rats administered with drugs was shown to be shorter than that of the rats of control group as seen in Figure 3. In particular, the moving time of the rats fed phentermine or mahuang was 30 or 22 sec, respectively, while that of rats of control group was above 50 sec, which means that the ability to maintain balancing of the rats administered with these drugs were significantly slowed down compared with that of control rats. Finally, spontaneous activities and exploratory behaviors of rats were evaluated in an open field test. The rats of control group were revealed to spend most time resting and grooming (Figure 4A). On the contrary, the mice fed phentermine, or mahuang were shown to spend most time moving slowly or fast as shown in Figure 4B & C. The duration of slow or fast movement by rats in an open field means that they are in a state of wariness or excitement not taking a rest. The retention time of the mice administered with phentermine, or mahuang in slow movement was significantly longer than that of control rats (Figure 4B). In addition, the retention time of the rats fed phentermine or mahuang in fast movement was also longer than that of control rats as demonstrated in Figure 4C. In particular, phentermine was shown to increase wariness or excitement keeping the mice from resting the most.
As anti-obesity medications, phentermine is mainly classified as an anorexing agent and mahuang a thermogenic agent. In the present study, we investigated the potential influences of phentermine and mahuang on behavioral changes employing animal experiments because they are considered to display effectiveness through the regulation of nerve system. Although some adverse side effects of phentermine and mahuang have been known, the studies on behavior changes by the influence of these drugs have been rarely performed. From the results of experiments testing locomotor activity through the use of treadmill, rota-rod, and open field, these drugs were commonly revealed to induce behavioral changes of mice by reducing a motor ability, an ability to cope with an external stimulus, and a sense of balance or by augmenting wariness or excitement.
Phentermine is a appetite suppressant and a currently marketed drug for short-term weight management in U.S., Korea, and some countries [15]. Even though Kang et al reported that any systematic adverse events with phentermine were not identified [22], they emphasized in other report that the possible side effects of phentermine such as insomnia, dry mouth, dizziness, palpitation, hand tremor, and elevation in blood pressure and pulse rate should be carefully considered because it has sympathomimetic properties [15]. Another report also insisted that the rate of serious adverse events could be as high as 15 per 1,000 of the patients who received phentermine [23]. In the present study, the rats ingested with phentermine showed a decreased motor ability on a moving treadmill compared to control rats. However, it was revealed that phentermine did not much influence on an ability to react to micro current stimulus and a sense of balance of rats. On the other hand, the rats ingested with phentermine represented the most an affective disturbance and an excitement not taking rest than any other rats in open field test.
Mahuang is a natural substance including mainly ephedrine and d-N-pseudoephedrine and has been used for anti-obesity and weight loss in the east and the west. According to a previous study, mahuang effectively decreased body weight, fasting glucose levels and insulin levels in healthy overweight and obese populations [24]. However, as the adverse side effects related with gastrointestinal, cardiovascular and autonomic nervous system were reported [25], the U.S. FDA prohibited the use of mahuang and ephedrine as food additives in 2004 (http://www.fda.gov/oc/initiatives/ephedra/february2004/finalsummary.html). This means the prohibition of an indiscreet use of mahuang and ephedrine in health supplements, but not of the application in medications. From the results of locomotion activity tests in the present study, mahuang was also shown to significantly reduce a motor ability, an ability to react to external stimuli, and a sense of balance of rats. However, mahuang did not induce the severe excitement in rats though alertness and anxiety were observed by the ingestion of mahuang.
Taken together, these results revealed that phenternime and mahuang induced a slowdown in locomotor activity, a loss in balancing and an increase in wariness or excitement. These adverse effects of phenternime and mahuang in behavioral changes might be cautiously forecasted in humans and therefore should be reflected in the prescription of these drugs. As stressed again, phentermine and mahuang should be prescribed for only obesity where it is anticipated that the benefits of the treatment outweigh these potential side effects. In addition, the mechanisms related with behavioral disturbances of these drugs should be investigated for the reduction of the adverse effects in further studies.
Acknowledgment
This study was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) of Korea government (2009-0094035).
References
1. Bianco AC, McAninch EA. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013; 1(3):250–258. PMID: 24622373.

2. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009; 53(21):1925–1932. PMID: 19460605.
3. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001; 409(6818):307–312. PMID: 11201732.

4. Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond). 2005; 29(10):1168–1174. PMID: 15925949.

5. Kelley GA, Kelley KS. Effects of exercise in the treatment of overweight and obese children and adolescents: a systematic review of meta-analyses. J Obes. 2013; 2013:783103. PMID: 24455215.

6. Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006; 5(11):919–931. PMID: 17080028.

7. Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006; 29(4):277–302. PMID: 16569079.

8. Trigueros L, Peña S, Ugidos AV, Sayas-Barberá E, Pérez-Álvarez JA, Sendra E. Food ingredients as anti-obesity agents: a review. Crit Rev Food Sci Nutr. 2013; 53(9):929–942. PMID: 23768185.

9. McClendon KS, Riche DM, Uwaifo GI. Orlistat: current status in clinical therapeutics. Expert Opin Drug Saf. 2009; 8(6):727–744. PMID: 19998527.

10. Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008; 31(1):53–65. PMID: 18095746.
11. Akbas F, Gasteyger C, Sjödin A, Astrup A, Larsen TM. A critical review of the cannabinoid receptor as a drug target for obesity management. Obes Rev. 2009; 10(1):58–67. PMID: 18721231.

12. Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D, Dosa PI, Covel JA, Ren A, Webb RR, Beeley NR, Martin M, Morgan M, Espitia S, Saldana HR, Bjenning C, Whelan KT, Grottick AJ, Menzaghi F, Thomsen WJ. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008; 51(2):305–313. PMID: 18095642.

13. Shyh G, Cheng-Lai A. New antiobesity agents: lorcaserin (Belviq) and phentermine/topiramate ER (Qsymia). Cardiol Rev. 2014; 22(1):43–50. PMID: 24304809.
14. Snow V, Barry P, Fitterman N, Qaseem A, Weiss K. Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005; 142(7):525–531. PMID: 15809464.

15. Kang JG, Park CY. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab J. 2012; 36(1):13–25. PMID: 22363917.

16. Abourashed EA, El-Alfy AT, Khan IA, Walker L. Ephedra in perspective--a current review. Phytother Res. 2003; 17(7):703–712. PMID: 12916063.
17. Song MK, Um JY, Jang HJ, Lee BC. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp Ther Med. 2012; 3(4):707–712. PMID: 22969956.

18. Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagné J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003; 289(12):1537–1545. PMID: 12672771.

19. Shekelle P, Hardy M. Safety and efficacy of ephedra and ephedrine for enhancement of athletic performance, thermogenesis and the treatment of obesity. Phytomedicine. 2002; 9(1):78. PMID: 11924769.
20. Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014; 130:47–53. PMID: 24657739.

21. Yang G, Park D, Lee SH, Bae DK, Yang YH, Kyung J, Kim D, Choi EK, Hong JT, Jeong HS, Kim HJ, Jang SK, Joo SS, Kim YB. Neuroprotective Effects of a Butanol Fraction of Rosa hybrida Petals in a Middle Cerebral Artery Occlusion Model. Biomol Ther (Seoul). 2013; 21(6):454–461. PMID: 24404336.

22. Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010; 12(10):876–882. PMID: 20920040.

23. Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005; 142(7):532–546. PMID: 15809465.

24. Hackman RM, Havel PJ, Schwartz HJ, Rutledge JC, Watnik MR, Noceti EM, Stohs SJ, Stern JS, Keen CL. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: a randomized controlled trial. Int J Obes (Lond). 2006; 30(10):1545–1556. PMID: 16552410.

25. Pittler MH, Ernst E. Dietary supplements for body-weight reduction: a systematic review. Am J Clin Nutr. 2004; 79(4):529–536. PMID: 15051593.

Figure 1
Measurement of the moving distance on a treadmill. Treadmill has a metal bar that provides a foot shock (0.4 mA micro current) that causes a tingling sensation. After rats were subjected to a running test on a treadmill at 15 cm/sec, the moving distance on a metal bar were measured during three-times of foot shock. Data are presented as mean±SEM. A value of P<0.05 (*) was considered to be statistically significant compared to control.

Figure 2
Measurement of a staying time on a treadmill. After rats were subjected to a running test on a treadmill at 15 cm/sec, the staying time which is the elapsed time for a rat to return to the front of a moving platform when a foot shock stimulation was applied to a rat was measured. Data are presented as mean±SEM. A value of P<0.05 (*) was considered to be statistically significant compared to control.
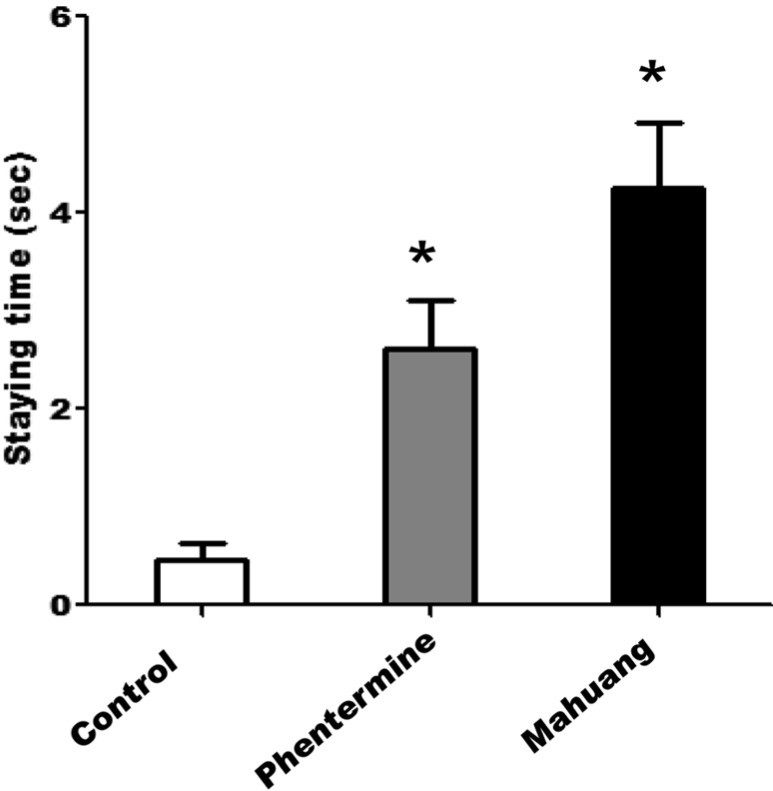
Figure 3
Measurement of a sense of motor coordination and balance using a rota-rod. Rats were subjected to a moving test on rota-rod test system of which rotation speed was accelerated from 5 rpm to 40 rpm within 30 sec in an early stage and maintained at 40 rpm afterwards. The time of falling off the rod was recorded for each mouse. Data are presented as mean±SEM. A value of P<0.05 (*) was considered to be statistically significant compared to control.
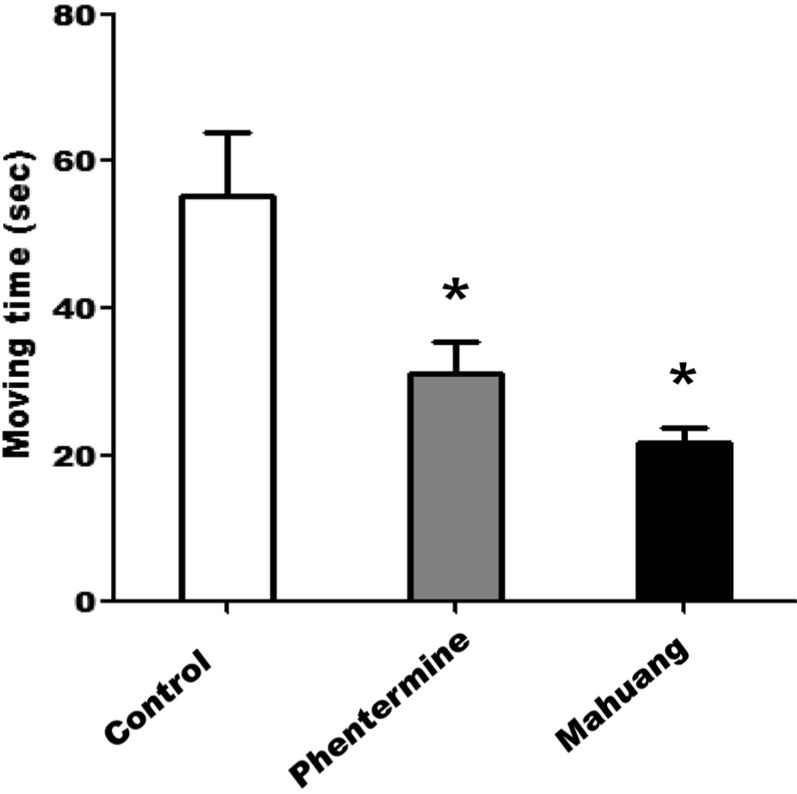
Figure 4
Measurement of spontaneous activities and exploratory behaviors in an open field. Mice were placed in a quiet black chamber with dim light, and the time spent for each type of movements, i.e., (A) resting (below 100 cm/sec), (B) slow-moving (100~300 cm/sec), and (C) fast-moving (300~500 cm/sec), was recorded for 5 min for each mouse. Time interval between movements was 5 sec. Data are presented as mean±SEM. A value of P<0.05 (*) was considered to be statistically significant compared to control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download