Abstract
To investigate the toxic effects of cheonggukjang (CKJ) manufactured using mixed cultures of Bacillus subtilis MC31 and Lactobacillus sakei 383 on the liver and kidney of ICR mice, an alteration on the related markers including body weight, organ weight, urine composition, liver pathology and kidney pathology were analyzed after oral administration at dosage of 25, 50 and 100 mg/kg body weight/day of CKJ for 14 days. Any significant toxicity was not observed on the body and organ weight, clinical phenotypes, urine parameters and mortality in the CKJ-treated group compared with the vehicle-treated group. Also, liver toxicity analysis revealed no significant increase in alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) or lactate dehydrogenase (LDH) in response to CKJ. Additionally, the specific pathological features induced by most toxic compounds were not observed upon liver histological analysis. Furthermore, kidney toxicological analysis revealed that blood urea nitrogen (BUN) and the serum creatinine (Cr) levels and pathological features on histological sections did not differ significantly between the vehicle- and CKJ-treated groups. Overall, these results suggest that CKJ does not induce any specific toxicity in liver and kidney organs of ICR at dose of 100 mg/kg body weight/day as no observed adverse effect level (NOAEL).
CKJ is produced during the short-term fermentation of soybeans using natural microflora such as Bacillus subtilis [1,2]. During CKJ fermentation, flavonoid glycosides are converted into aglycones by hydrolysis, and many proteins are degraded into small peptides and amino acids [3,4]. Therefore, the final CKJ product contains many enzymes, microorganisms, and bioactive compounds that are absent from unfermented soybeans; accordingly, it is considered a good source of protein, hydrolyzed peptides and lipids [1,2,5].
Anti-obesity, anti-diabetic, anti-oxidative, anti-hypertensive and anti-inflammatory effects of CKJ have been investigated by various research groups. The results of these studies indicated that CKJ supplementation in human subjects significantly reduced visceral fat mass and apolipoprotein B/apolipoprotein A1 levels [6], while C57BL/6J mice exhibiting diet-induced obesity showed improvements in body weight, epididymal fat accumulation, serum total cholesterol, and LDL-cholesterol [7]. Furthermore, significant reduction in blood glucose and glycoslylated hemoglobin levels and improved insulin tolerance were observed in C57BL/Ksj-db/db mice, after CKJ administration [8,9]. CKJ treatment was also found to decrease passive cutaneous anaphylaxis in rat models of type I hypersensitivity and arachidonic acid-induced ear edema [10]. Ethanol extracts of CKJ have been shown to significantly increase the viability of cultured mice spleen and thymus cells by suppressing apoptotic death [11]. Additionally, CKJ was reported to induce recovery of nerve growth factor (NGF) level and the phosphorylation level of TrkA and Erk in the NGF receptor TrkA signaling pathway in Tg2576 mice expressing AD phenotypes [12]. CKJ extract may also enhance sensitivity of the femur, liver, muscle and epiphyseal growth plate in SD rats through enhancement of GH secretion [13]. Furthermore, other studies have investigated the toxicity of some soybean related products including fermented corticated soybean meal (FCSBM), touchi extract (TE), and Korea soybean paste (doen-jang) [14,15,16]. Despite the increased attention the therapeutic effects of CKJ have received, its toxicity against specific target organs of mice have not been investigated to date. Therefore, in this study, we investigated the effects of CKJ on several toxic indicators of ICR mice following short-term treatment. The results show that there is a scientific basis for determining the optimal concentration of CKJ as functional food for the improvement of human chronic diseases.
CKJ was prepared as previously described [12,13]. The soybean (Daepung strain) used to manufacture CKJ was kindly supplied by the National Institute of Crop Science in Miryang, Korea, while B. subtilis MC31 and L. sakei 383 were obtained from the Food Microbiology Laboratory at Pusan National University. To manufacture CKJ extract, 30 g of soybeans were washed and soaked with three volumes of tap water at room temperature for 12 h. The soybeans were then treated with hot steam at 121℃ for 30 min and allowed to cool to 40℃, after which they were inoculated with 1% (w/w) B. subtilis MC31 and L. sakei 383 and fermented for 72 h at 37℃. Finally, the fermented soybeans were powdered through several steps including freeze-drying, homogenization and sifting. The final sample of CKJ extract was stored at -75℃ until use.
GABA concentration was measured by a spectrophotometric assay containing GABase enzyme (Sigma-Aldrich, St. Louis, MO, USA) as described by Zhang and Bown [17]. First, 0.3 g of lyophilized powder was dissolved in 99% ethanol (1.2mL) for 5 h. The supernatant (0.1 mL) was then mixed with 0.4 mL of MeOH and completely dried at 70-80℃ for 30 min. Next, 70 mM LaCl3 (1 mL) was added and the mixture was shaken for 10 min. The sample was then centrifuged at 10,000 rpm for 5 min, after which the supernatant (0.8 mL) was mixed with 0.1 M KOH solution (0.16 mL) for 3-5 min. This solution was then further purified by centrifugation and filtration for subsequent enzyme reaction. Finally, this solution (0.55 mL) of CKJ was dispensed into individual cuvettes that each contained 0.2 mL of 0.5 mM K4P2O7 buffer (pH 8.6), 0.15 mL of 4 mM NADP, and 0.05 mL of GABase (2 unit/mL). The initial absorbance was then read at 340 nm using a spectrophotometer (Optizen POP, Mecasys Co., Ltd., Daejeon, Korea), after which 0.05 mL of 20 mM α-ketoglutarate was added and the samples were incubated at 60 min at room temperature, at which time the final absorbance was read at the same wavelength. The final concentration of GABA was then calculated by comparison of the difference between the two absorbances and by comparison with a standard curve.
The concentration of diadzein and genistein in CKJ was measured by dissolving aqueous extract of CKJ in 50% MeOH at 100 mg/mL while shaking at 200 rpm for 4 h. Following incubation for 12 h at room temperature, the mixture was centrifuged at 3,000 rpm, after which the supernatant was harvested, diluted to 25 mg/mL in 50% MeOH and passed through a syringe filter (0.45 µm).
CKJ was analyzed using an iLC 3000 HPLC system (Interface Engineering, Seoul, Korea) equipped with a Corona® CAD® Detector (ESA Bioscience, Inc., Chelmsford, MA, USA). Chromatographic separation was performed using a YMC-triart C18 column (4.6 mm ×250 mm, particle size 5 µm, Shiseido Co., Ltd., Tokyo, Japan). The mobile phase consisted of solvent A (0.1% formic acid in deionized water) and solvent B (acetonitrile). Samples were subjected to the following gradient elution program: 0-30 min, 20-40% of solvent B and 30-45 min, 40-70% of solvent B. A flow rate of 1.0 mL/min was used for the sample analysis and the nebulizer gas was nitrogen. The gas flow rate and pressure were maintained at 1.53 L/min and 35±2 psi, respectively. The output signal of the detector was recorded using the Clarity™ chromatography software (DataApex, Prague, Czech Republic).
The animal protocol used in this study was reviewed and approved based on the ethical and scientific care procedures of the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2013-0465). The animals were handled in the Pusan National University-Laboratory Animal Resources Center accredited by AAALAC International (Accredited Unit Number-001525) in accordance with the USA NIH guidelines and the Korea Food and Drug Administration (KFDA; Accredited Unit Number-00231) in accordance with the Laboratory Animals Act. Female ICR mice were purchased from Samtako (Osan, Korea) and provided with standard irradiated chow diet (Purina Mills Inc., Seoungnam, Korea) ad libitum. All mice were and maintained in a specific pathogen-free state under a strict light cycle (12 h light-dark cycle) at a temperature of 22±2℃ and 50±10% relative humidity.
Eight-week-old ICR mice (n=40) were assigned to one of the following four groups: vehicle-treated group (n=10), low dosage CKJ-treated group (n=10), medium dosage CKJ-treated group (n=10), and high dosage CKJ-treated group (n=10). As a control, one group of ICR mice received a comparable volume of daily water via gavage (vehicle-treated group), whereas the others received 25 mg/kg body weight/day of CKJ (low dosage CKJ-treated group), 50 mg/kg body weight/day of CKJ (medium dosage CKJ-treated group), or 100 mg/kg body weight/day of CKJ (high dosage CKJ-treated group) via gavage. The concentration of CKJ was determined from the results of previous studies which 25-100 mg/kg of CKJ administration lead to reducing anaphylaxis, increasing memory ability and suppressing asthma [18, 19, 20]. Also, the administration period for toxicity test was citied the Guideline for Drug Toxicity published from Korea Food and Drug Administration. After CKJ treatment for 14 days, all mice were immediately sacrificed using CO2 gas, after which the urine, blood and tissue samples were prepared.
Clinical signs and the number of animals that died were recorded more than twice a day for 14 days. In addition, alterations in body weight were observed using an electronic balance (Mettler Toledo, Greifensee, Switzerland) every day according to the KFDA guidelines. Finally, the weights of nine organs (brain, ovary, testis, kidney, spleen, liver, thymus, heart and lung) collected from the sacrificed mice were determined using the same method employed to detect the body weight.
All mice were sacrificed using CO2 gas at 24 h after the final administration, which urine was collected from their bladders and assayed for bilirubin, urobilinogen, ketones, protein, pH, specific gravity and leucocytes with an urine analyzer URiSCAN optima II (Yeongdong Electronics Co., Ltd., Yongin, Korea). All assays were conducted in triplicate using fresh urine.
After fasting for 8 h, whole blood of each mouse in all groups was collected from their abdominal veins and incubated for 30 min at room temperature. Serum was then obtained by centrifugation of blood and analyzed for ALP, ALT, AST, LDH, BUN, and Cr using an Automatic Serum Analyzer (HITACHI 747, Tokyo, Japan). All assays were conducted in triplicate using fresh serum.
Liver and kidney tissues collected from ICR mice were fixed with 10% formalin for 12 h, embedded in paraffin wax, and sectioned into 4 µm slices. Next, liver and kidney sections were stained with hematoxylin and eosin (H&E, Sigma-Aldrich), after which pathological changes were measured using Leica Application Suite (Leica Microsystems, Heerbrugg, Switzerland).
Significant differences between vehicle- and CKJ-treated ICR mice were identified by one-way analysis of variance (ANOVA) using SPSS for Windows, Release 10.10, Standard Version (SPSS Inc., Chicago, IL, USA). All values are reported as the mean±standard deviation (SD). A P value<0.05 was considered to be significant.
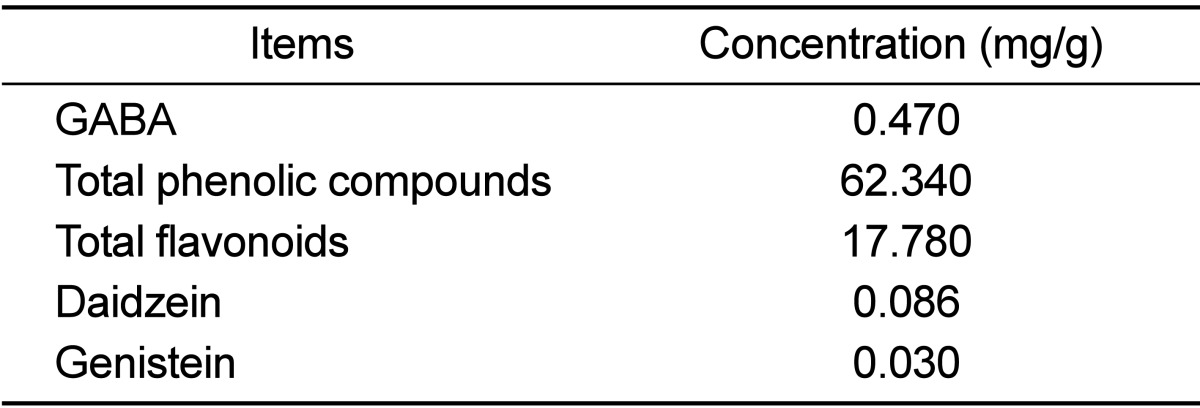
To observe the distribution of key components, the concentration of GABA, total phenolic compounds and total flavonoids, isoflavones (diadzein and genistein) were measured in CKJ extracts. As shown in Table 1, the concentration of GABA was 0.470 mg/g. Additionally, total phenolic compounds and total flavonoids in CKJ extract were found to be 62.340 and 17.780 mg/g, respectively. Furthermore, the level of the daidzein and genistein were 0.086 and 0.030 mg/g, respectively. Therefore, these results demonstrate that CKJ extract contained high concentrations of GABA and phenolic compounds, but low concentrations of isoflavones.
To examine the phenotypical toxicity induced by CKJ treatment, the clinical phenotypes and mortality were observed in ICR mice over 14 days. Mice treated with CKJ did not show any significant pathological symptoms such as melancholy, hypokinesia, gait abnormality, or tremors. Furthermore, no dead mice were observed in any CKJ treatment groups (data not shown). These data indicate that CKJ does not induce any significant changes on pathological symptoms or mortality of ICR mice.
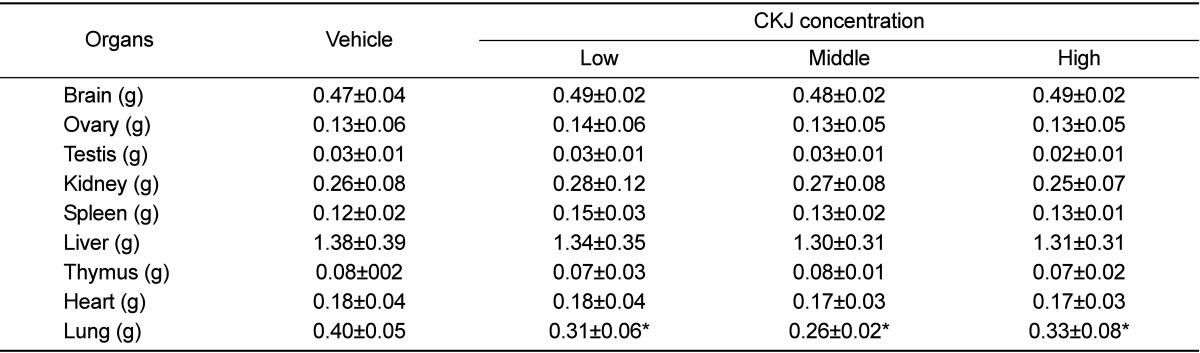
Generally, alterations in body and organ weights of animals are considered to be indicators of animal toxicity. Therefore, the toxicity of CKJ was measured in CKJ treated mice based on body and organ weight. No significant alterations of body weight were observed in any of the CKJ-treated groups compared to the vehicle-treated group throughout the experimental period (Figure 1). Additionally, no changes in the brain, heart, liver, kidney, spleen, thymus, testis or ovary morphology or weight were observed in the vehicle-treated, low, medium, and high dosage CKJ-treated groups although lung weight was significantly decreased in groups treated with three different dosages of CKJ compared to the vehicle-treated group. Furthermore, no pathological signs were observed in any of the organs in all treatment groups (Table 2). These results suggest that CKJ treatment does not induce any toxic alterations on organ weights of ICR mice.
The levels of seven urine factors were measured in ICR mice. Bilirubin, urobilinogen, protein, pH, specific gravity and leucocytes in urine showed no change in concentration in any groups although the level of ketones was significantly higher in the CKJ-treated group than the vehicle-treated group (Figure 2). These results indicate that CKJ did not exert any toxic alterations on urine toxic parameters of ICR mice.
To examine CKJ toxicity in liver organs of ICR mice, the levels of several enzymes related to liver metabolism were measured in blood serum. No increase in ALP, AST, ALT, and LDH were observed in response to any doses of CKJ, although they did decrease slightly in some groups (Figure 3A). Additionally, evaluation of liver sections stained with H&E revealed no significant pathological features such as inflammation, necrosis, bilirubin accumulation, or iron deposition in any groups (Figure 3B). Therefore, these results suggest that CKJ treatment for short-term does not induce toxic effects in the livers of ICR mice.
Kidney toxicity against CKJ treatment was investigated in ICR mice by serum biochemical and histological analyses. The level of BUN and Cr was not altered significantly in any CKJ-treated group (Figure 4A). Furthermore, no specific pathological symptoms were detected in any of the CKJ-treated groups, and most kidney cells maintained their normal structures. Degeneration and necrosis of the glomerulus and renal tubes induced by toxicants and immunological factors were not observed in any region of the kidney, and no edema or swelling were observed in the renal tubes of kidney tissue (Figure 4B). These results suggest that short-term CKJ does not induce specific toxic effects in the kidneys of ICR mice.
Traditionally, CKJ is prepared through fermentation of steamed soybeans with B. subtilis at about 40℃ over 2 to 3 days, while doen-jang requires a much longer fermentation period under the same conditions [21]. Many suitable fermentative Bacillus species have recently been recovered from CKJ or CKJ-related products, and functional activities of soy foods fermented with Bacillus species may be associated with total contents and/or types of various components such as isoflavones [14,22,23]. To identify suitable fermentative bacteria, we prepared CKJ using B. subtilis MC31 and L. sakei 383. This product was found to have a higher concentration of GABA, which acts as a neurotransmitter, than traditional CKJ. However, the present study is the first investigation of whether CKJ induces toxicity in specific organs of ICR mice.
Alteration of body and organ weight is considered a primary indicator of toxicity for various substances in the toxicological evaluation process [24]. Among several organs, the liver and kidney are considered major target organs since they contain the most enzymes and metabolic pathways for xenobiotic excretion [25]. A significant increase in liver weight can further progress to abnormal fat accumulation and obesity, as well as necrosis of liver cells [26,27,28,29,30,31]. However, no significant toxicity was observed in cells or animals treated with various types of soybean related products [15,16]. Three different doses of TE obtained from soybeans fermented with Aspergillus oryzae did not induce any significant effects on clinical signs, body weight, food consumption, urinalysis, hematology, blood chemistry, necropsy, organ weight or histology [15]. Furthermore, the water extract from doen-jang did not exhibit cytotoxicity or mutagenicity on Chinese hamster lung cells or Salmonella typhimurium strains [16]. In this study, no toxic alteration of organ weight was observed in any of the CKJ-treated groups although lung weight was decreased in CKJ-treated groups. These findings are mostly in agreement with the above reports. In addition, the lung toxicity has been induced by the complex mechanism including a direct cytotoxicity and an indirect immune response although the exact causes are unknown [32]. The weight of this organ could be enhanced with the accumulation of lipid in lung cells during the immune response, while it could be decreased after contracting the some diseases including diaphragmatic hernia, dysostosis and hydrocephalus [33]. In our study, the decrease of lung weight was observed in all CKJ-treated groups compared with vehicle-treated group. However, it is not quite sure that this response has directly associated with CKJ toxicity, because any significant pathological symptoms were not detected in the lung tissue during necropsy as well as this response may be induced by a number of factors. Especially, some interesting evidences for this alteration were suggested by the previous study, which treatment with CKJ induced the reducing the number of eosinophils and monocytes in the lungs of mice and suppressed histoathological alterations including eosinophils infiltration, mucus accumulation, hyperplasia of goblet cells and deposition of collagen fiber [34].
Ketones (acetoacetate, 3-b-hydroxybutyrate and acetone) are majorly produced from fatty acid in the liver and used as an energy source when there is limited availability of carbohydrate or when carbohydrate cannot be used effectively [35]. Also, the levels of circulating ketones are very diverse in populations of normal individuals even after controlling for age and period of fasting [35]. This variation is presumably caused by differences in basal metabolic rate, hepatic glycogen stores as well as variations in the mobilization of amino acids from muscle proteins [36]. The level of ketone body ratio can also be markedly changed by abnormal food or nutrition, disorders of increased metabolism, acute or severe illness, burns, fever, hyperthyroidism, nursing a baby and pregnancy [35,36,37,38,39]. Especially, abnormal food or nutrition intake is mainly due to several factors including anorexia, fasting, high protein or low carbohydrate diets, starvation and vomiting over a long period of time [37]. Therefore, it is likely that the increase of ketone level observed in our study can be majorly attributed to the administration of abnormal food or nutrition because CKJ have contained higher protein than carbohydrate. However, we proposed that the detail mechanism should be investigated to determine if CKJ treatment are actually enhanced ketone body.
Alteration of the level of four enzymes including ALT, AST, ALP and LDH is commonly used as a marker of altered liver toxicity and health. Among these enzymes, ALT is found in the serum as well as various tissues [30], while AST is found in the liver, heart, skeletal muscle, kidneys, brain, and red blood cells [29]. These two enzymes are released into the blood when liver cells are disrupted by various factors [29,30]. In this study, CKJ treatment did not induce any significant alteration on the level of these two enzymes. The level of ALP and LDH can be used to diagnose liver disease or bone disorders [31]. ALP activity increases under conditions of bile secretion disease, which include primary biliary cirrhosis as well as extra- and intra-hepatic cholestasis, while LDH is present in almost all animal tissues and up-regulated in response to cell damage [40,41]. In this study, we used these four factors to investigate CKJ toxicity toward the livers of mice. The results showed that CKJ did not have any toxic effects on liver tissue of CKJ-treated mice based on the serum levels of these enzyme indicators. Furthermore, non-toxicity of CKJ in mice suggested that CKJ did not contain any toxic compounds, although it contained various functional compounds including GABA, diadzein and genistein. Moreover, massive coagulative necrosis in the central vein as well as degenerative changes with fatty alteration of the necrotic border region can be induced by liver toxicants such as CCl4 [40]; however, no such changes were observed in the present study.
Kidney toxicity was also evaluated based on alteration of the serum levels of BUN and Cr, which are known to increase dramatically in response to kidney trauma and to show an increased ratio upon upper gastrointestinal tract bleeding, hemocytotripsis, inflammation, administration of certain drugs and fever [41,42]. In this study, we measured the levels of BUN and Cr after CKJ treatment to evaluate CKJ toxicity in the kidney and found that they were maintained at a constant level. Additionally, pathological alterations such as necrosis of the proximal tube in kidney tissue were observed in mice treated with paraquat (1'1'-dimetythyl-4'4'-bipyridyliumion) [43]; however, no such changes were observed in the present study.
Taken together, these results demonstrate that CKJ prepared with coculture of B. subtilis MC31 and L. sakei 383 did not have any specific effects on most toxicological factors of ICR mice and NOAEL of CKJ was established 100mg/kg body weight/day. Furthermore, these data provide vital information regarding application of CKJ as a functional food with beneficial effects on several chronic diseases.
Acknowledgment
This study was supported by grants to Dr. Dae Youn Hwang from the Korea Institute of Planning Evaluation for Technology of Food, Agriculture, Forestry and Fisheries (110119-3).
References
1. Lee JJ, Lee DS, Kim HB. Fermentation patterns of chungkookjang and Kanjang by Bacillus licheniformis B1. Korean J Microbiol. 1999; 35(4):296–301.
2. Su CL, Wu CJ, Chen FN, Wang BJ, Sheu SR, Won SJ. Supernatant of bacterial fermented soybean induces apoptosis of human hepatocellular carcinoma Hep 3B cells via activation of caspase 8 and mitochondria. Food Chem Toxicol. 2007; 45(2):303–314. PMID: 17030378.

3. Nakajima N, Nozaki N, Ishihara K, Ishikawa A, Tsuji H. Analysis of isoflavone content in tempeh, a fermented soybean, and preparation of a new isoflavone-enriched tempeh. J Biosci Bioeng. 2005; 100(6):685–687. PMID: 16473782.

4. Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH, Park S. The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-gamma activity in vitro. Biofactors. 2006; 26(4):245–258. PMID: 17119271.
5. Lee SJ, Rim HK, Jung JY, An HJ, Shin JS, Cho CW, Rhee YK, Hong HD, Lee KT. Immunostimulatory activity of polysaccharides from Cheonggukjang. Food Chem Toxicol. 2013; 59:476–484. PMID: 23831309.

6. Back HI, Kim SR, Yang JA, Kim MG, Chae SW, Cha YS. Effects of Chungkookjang supplementation on obesity and atherosclerotic indices in overweight/obese subjects: a 12-week, randomized, double-blind, placebo-controlled clinical trial. J Med Food. 2011; 14(5):532–537. PMID: 21434780.
7. Soh J, Kwon DY, Cha YS. Hepatic gene expression profiles are altered by dietary unsalted Korean fermented soybean (chongkukjang) consumption in mice with diet-induced obesity. J Nutr Metab. 2011; 2011:260214. PMID: 21437188.

8. Kim DJ, Jeong YJ, Kwon JH, Moon KD, Kim HJ, Jeon SM, Lee MK, Park YB, Choi MS. Beneficial effect of chungkukjang on regulating blood glucose and pancreatic beta-cell functions in C75BL/KsJ-db/db mice. J Med Food. 2008; 11(2):215–223. PMID: 18598161.
9. Kwon DY, Daily JW 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010; 30(1):1–13. PMID: 20116654.

10. Choi YH, Lim H, Heo MY, Kwon DY, Kim HP. Anti-inflammatory activity of the ethanol extract of Chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J Med Food. 2008; 11(3):539–543. PMID: 18800904.

11. Kim HB, Lee HS, Kim SJ, Yoo HJ, Hwang JS, Chen G, Youn HJ. Ethanol extract of fermented soybean, Chungkookjang, inhibits the apoptosis of mouse spleen, and thymus cells. J Microbiol. 2007; 45(3):256–261. PMID: 17618232.
12. Lee YJ, Kim JE, Kwak MH, Go J, Son HJ, Kim DS, Hwang DY. In vitro and in vivo study of effects of fermented soybean product (chungkookjang) on NGF secretion ability and NGF receptor signaling pathway. Lab Anim Res. 2013; 29(2):113–126. PMID: 23825484.
13. Hwang IS, Kim JE, Lee YJ, Kwak MH, Lee HG, Kim HS, Lee HS, Hwang DY. Growth sensitivity in the epiphyseal growth plate, liver and muscle of SD rats is significantly enhanced by treatment with a fermented soybean product (cheonggukjang) through stimulation of growth hormone secretion. Mol Med Rep. 2014; 9(1):166–172. PMID: 24173540.

14. Wongputtisin P, Khanongnuch C, Khongbantad W, Niamsup P, Lumyong S. Screening and selection of Bacillus spp. for fermented corticate soybean meal production. J Appl Microbiol. 2012; 113(4):798–806. PMID: 22788990.

15. Fujita H, Yamagami T. Absence of mutagenicity, genotoxicity, and subchronic oral toxicity of Touchi extract. Int J Toxicol. 2007; 26(5):465–473. PMID: 17963133.

16. Kim JG. Antigenotoxic effects of water extract from Korean fermented soybean paste (doen-jang). J Food Prot. 2004; 67(1):156–161. PMID: 14717366.

17. Zhang G, Bown AW. The rapid determination of γ-aminobutyric acid. Phytochemistry. 1997; 44(6):1007–1009.

18. Choi YH, Lim H, Heo MY, Kwon DY, Kim HP. Anti-inflammatory activity of the ethanol extract of Chungkukjang, Korean fermented bean: 5-lipoxygenase inhibition. J Med Food. 2008; 11(3):539–543. PMID: 18800904.

19. Kim JH. Protective effects of purple sweet potato added to Bacillus subtilis-fermented soymilk against amyloid beta-induced memory impairment. J Agric Sci. 2012; 4(4):223–232.

20. Bae MJ, Shin HS, See HJ, Chai OH, Shon DH. Cheonggukjang ethanol extracts inhibit a murine allergic asthma via suppression of mast cell-dependent anaphylactic reactions. J Med Food. 2014; 17(1):142–149. PMID: 24456365.

21. Yang SO, Chang PS, Lee JH. Isoflavone distribution and â-glucosidase activity in cheonggukjang, a traditional Korean whole soybean-fermented food. Food Sci Biotechnol. 2006; 15(1):96–101.
22. Kindoli S, Lee HA, Kim JH. Properties of Bac W42, a bacteriocin produced by Bacillus subtilis W42 isolated from Cheonggukjang. J Microbiol Biotechnol. 2012; 22(8):1092–1100. PMID: 22713985.

23. Lee NK, Yeo IC, Park JW, Kang BS, Hahm YT. Isolation and characterization of a novel analyte from Bacillus subtilis SC-8 antagonistic to Bacillus cereus. J Biosci Bioeng. 2010; 110(3):298–303. PMID: 20547349.

24. Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007; 168(2):176–185. PMID: 17197136.

25. Yun TK, Lee YS, Kwon HY, Choi KJ. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996; 17(4):293–298. PMID: 9812705.
26. Ryu KS, Lee HS, Kim SY. Effects of Bombyx mori larvae extracts on carbon tetrachloride-induced hepatotoxicity in mice. J Life Sci. 1999; 9(4):375–381.
27. Stripp B, Hamrick ME, Gillete JR. Effect of 3-methylcholanthrene induction on the carbon tetrachloride-induced changes in rat hepatic microsomal enzyme system. Biochem Pharmacol. 1972; 21(5):745–747. PMID: 5021594.

28. Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967; 19(2):145–208. PMID: 4859860.
29. You AS, Jeong MH, Park KH, Kim BS, Lee JB, Choi JH, Kwon OK, Kim JH. Effect on antioxidant function of onion to reduce pesticides toxicity. Korean J Pestic Sci. 2007; 11(4):222–229.
30. Bergmeyer HU. Methods of enzymatic analysis. 2nd ed. Academic Press: Weinheim;1974. p. 20.
31. Yoon SH, Park EJ, Oh KH, Chung YG, Kwon OJ. The effect of Lithospermi radix on benzo(a)pyrene-induced hepatotoxicity. J Korean Soc Food Nutr. 1993; 22(2):144–148.
32. Askenazi SS, Perlman M. Pulmonary hypoplasia: lung weight and radial alveolar count as criteria of diagnosis. Arch Dis Child. 1979; 54(8):614–618. PMID: 507916.

33. Chung JG, Cho YH, Joo SK, Seon HG, Kim EK, Jeong HC, Lee JH. Amiodarone-induced pulmonary toxicity with hemoptysis and a lung mass. Korean J Med. 2009; 77(5):S1202–S1205.
34. Bae MJ, Shin HS, See HJ, Chai OH, Shon DH. Cheonggukjang ethanol extracts inhibit a murine allergic asthma via suppression of mast cell-dependent anaphylactic reactions. J Med Food. 2014; 17(1):142–149. PMID: 24456365.

35. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999; 15(6):412–426. PMID: 10634967.

36. Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, Lambert M, Lapierre P, Potier E. Medical aspects of ketone body metabolism. Clin Invest Med. 1995; 18(3):193–216. PMID: 7554586.
37. Inzucchi SE, Sherwin RS. Type 1 diabetes mellitus. In : Goldman L, Schafer AI, editors. Cecil Medicine. 24th ed. Philadelphia: Saunders Elsevier;2011. chap 247.
38. Cahill GF Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA Jr, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966; 45(11):1751–1769. PMID: 5926444.

39. Terada Y, Eguchi Y, Chang YJ, Tabata R, Sakumoto H, Takehiro O, Uno S, Ozawa K. Ketone body ratios of the superior and inferior vena cava and of pulmonary arterial blood compared to that of arterial blood: central venous ketone body ratio as a substitute for the arterial ketone body ratio. Clin Chim Acta. 1996; 247(1-2):81–88. PMID: 8920229.

40. Hayes AW. Principles and methods of toxicology. 2nd ed. New York: Raven Press;1982. p. 447–474.
41. Kim DH, Deung YK, Lee YM, Yoon YS, Kwon KR, Park DB, Park YK, Lee KJ. The liver protecting effect of Pomegranate (Punica granatum) seed oil in mice treated with CCl4. Korean J Electron Microsc. 2006; 36(3):173–182.
42. Horiguchi H1, Oguma E, Kayama F, Sato M, Fukushima M. Dexamethasone prevents acute cadmium-induced hepatic injury but exacerbates kidney dysfunction in rabbits. Toxicol Appl Pharmacol. 2001; 174(3):225–234. PMID: 11485383.

43. Van De Graaff KM. Human anatomy. 6th ed. New York: McGraw-Hill;2002. p. 93–113.
Figure 1
Alteration of body weights of ICR mice. The weight of the whole body was measured daily using an electronic balance for 14 days. Data represent the means±SD from three replicates.
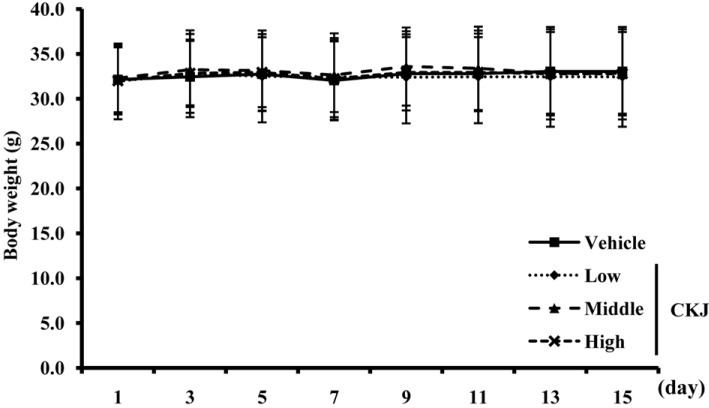
Figure 2
Alteration of urine parameters. After final administration of CKJ, urine was collected from the bladder of ICR mice using a syringe and the levels of seven factors were then analyzed as described in the Materials and Methods. Data represent the means±SD from three replicates. *P<0.05 indicates a significant difference compared to the vehicle-treated group.
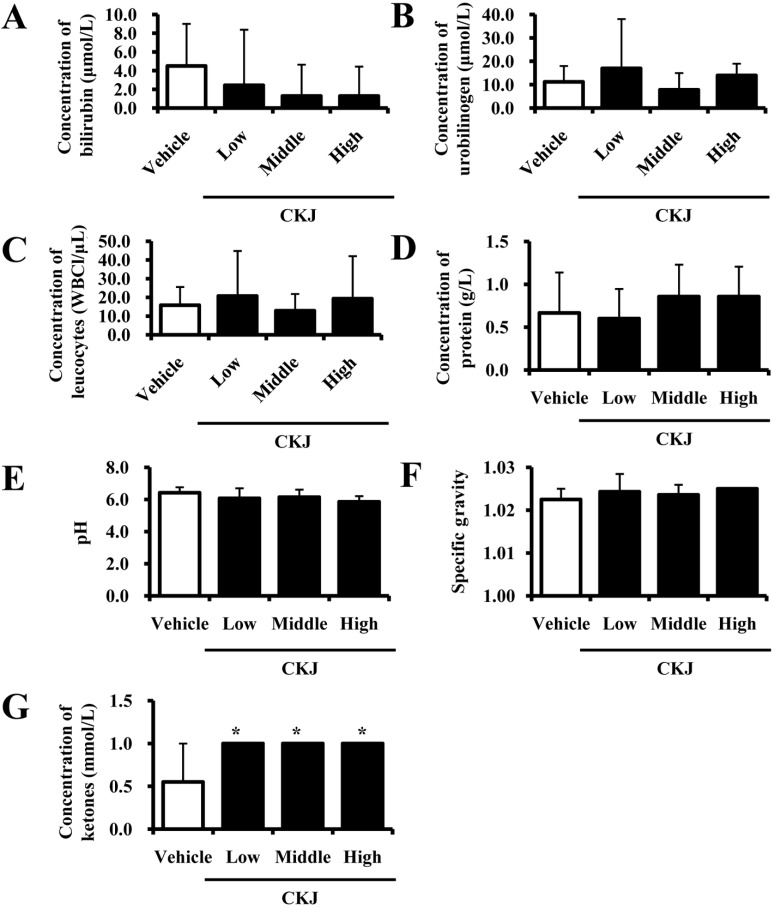
Figure 3
Liver toxicity in ICR mice. After final CKJ administration, blood was collected from the abdominal veins of vehicle- and CKJ-treated mice and serum concentrations of ALP (Aa), AST (Ab), ALT (Ac), and LDH (Ad) were then analyzed as described in the Materials and Methods. (B) Liver tissue of ICR mice was prepared on a histological slide and the cellular morphology was viewed at 400× magnification. Data represent the means±SD from three replicates. *P<0.05 indicates a significant difference compared to the vehicle-treated group.
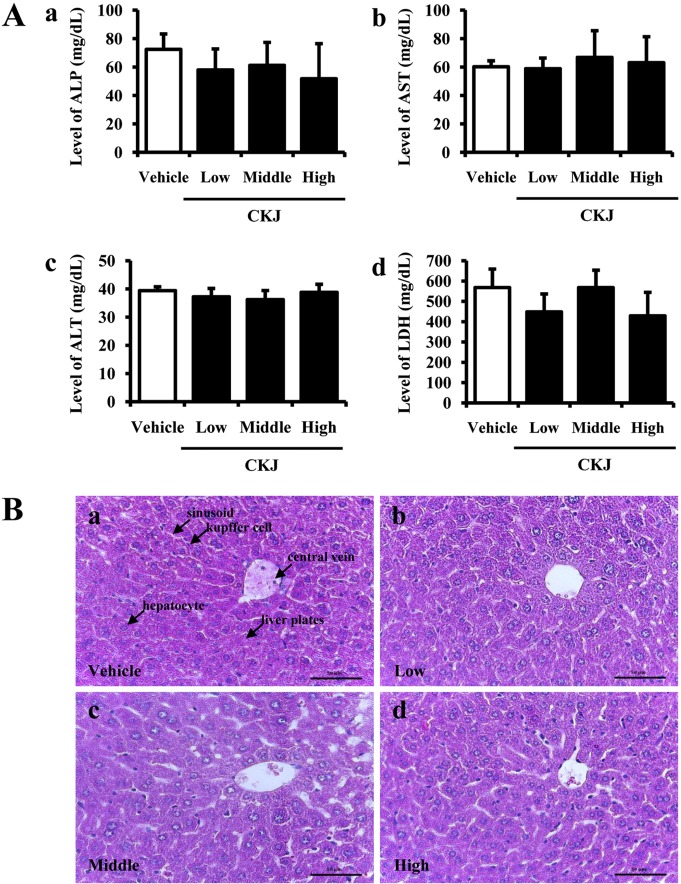
Figure 4
Kidney toxicity in ICR mice. After final CKJ administration, blood was collected from abdominal veins of vehicle- and CKJ-treated mice and serum concentrations of BUN (Aa) and Cr (Ab) were analyzed in duplicate as described in the Materials and Methods. (B) Cortex and medulla regions of kidney tissue of ICR mice were prepared on a histological section and the cellular morphology was viewed at 400× magnification. Data represent the means±SD from three replicates. *P<0.05 indicates a significant difference compared to the vehicle-treated group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download