1. Stokbroekx RA, Vandenberk J, Van Heertum AH, Van Laar GM, Van der Aa MJ, Van Bever WF, Janssen PA. Synthetic antidiarrheal agents. 2,2-Diphenyl-4-(4'-aryl-4'-hydroxypiperidino) butyramides. J Med Chem. 1973; 16(7):782–786. PMID:
4725924.
2. Hanauer SB. The role of loperamide in gastrointestinal disorders. Rev Gastroenterol Disord. 2008; 8(1):15–20.
3. Vandenbossche J, Huisman M, Xu Y, Sanderson-Bongiovanni D, Soons P. Loperamide and P-glycoprotein inhibition: assessment of the clinical relevance. J Pharm Pharmacol. 2010; 62(4):401–412. PMID:
20604828.

4. Katzung BG. Basic and Clinical Pharmacology. 9th ed. New York: McGraw-Hill Companies Inc.;2004. p. 321–370.
5. Wintola OA, Sunmonu TO, Afolayan AJ. The effect of
Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010; 10:95. PMID:
20723249.

6. Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS, Li B, Lee GH, Sung MS, Ha KC, Back HI, Kim SY, Park SH, Oh MR, Kim MG, Jeon JY, Im YJ, Hwang MH, So BO, Shin SJ, Yoo WH, Kim HR, Chae HJ, Chae SW. Effects of
Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol. 2012; 50(3-4):895–902. PMID:
22178225.
7. Méité S, Bahi C, Yéo D, Datté JY, Djaman JA, N'guessan DJ. Laxative activities of Mareya micrantha (Benth.) Müll. Arg. (Euphorbiaceae) leaf aqueous extract in rats. BMC Complement Altern Med. 2010; 10:7. PMID:
20158903.

8. Hughes S, Higgs NB, Turnberg LA. Loperamide has antisecretory activity in the human jejunum
in vivo. Gut. 1984; 25(9):931–935. PMID:
6590431.
9. Sohji Y, Kawashima K, Shimizu M. Pharmacological studies of loperamide, an anti-diarrheal agent. II. Effects on peristalsis of the small intestine and colon in guinea pigs (author's transl). Nihon Yakurigaku Zasshi. 1978; 74(1):155–163. PMID:
640534.
10. Yamada K, Onoda Y. Comparison of the effects of T-1815, yohimbine and naloxone on mouse colonic propulsion. J Smooth Muscle Res. 1993; 29(2):47–53. PMID:
8318729.

11. Yang ZH, Yu HJ, Pan A, Du JY, Ruan YC, Ko WH, Chan HC, Zhou WL. Cellular mechanisms underlying the laxative effect of flavonol naringenin on rat constipation model. PLoS One. 2008; 3(10):e3348. PMID:
18833323.

12. Bustos D, Ogawa K, Pons S, Soriano E, Bandi JC, Bustos Fernández L. Effect of loperamide and bisacodyl on intestinal transit time, fecal weight and short chain fatty acid excretion in the rat. Acta Gastroenterol Latinoam. 1991; 21(1):3–9. PMID:
1811403.
13. Robertson DG, Watkins PB, Reily MD. Metabolomics in toxicology: preclinical and clinical applications. Toxicol Sci. 2011; 120(Suppl 1):S146–S170. PMID:
21127352.

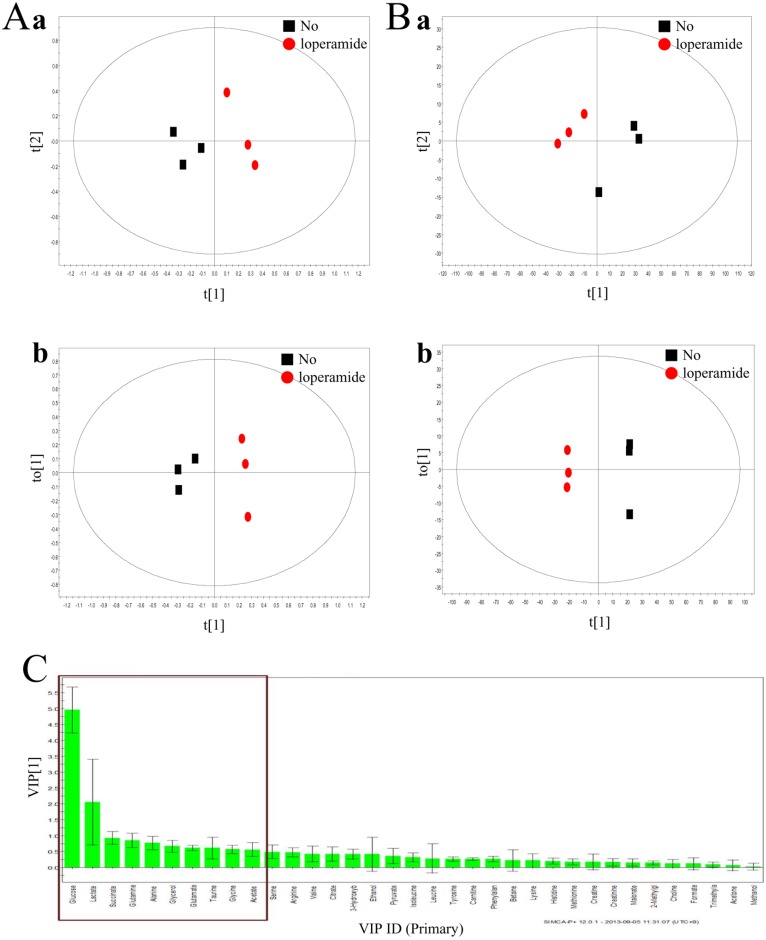
14. Kim KB, Chung MW, Um SY, Oh JS, Kim SH, Na MA, Oh HY, Cho WS, Choi KH. Metabolomics and biomarker discovery: NMR spectral data of urine and hepatotoxicity by carbon tetrachloride, acetaminophen, and d-galactosamine in rats. Metabolomics. 2008; 4:377–392.

15. Kim KB, Um SY, Chung MW, Jung SC, Oh JS, Kim SH, Na HS, Lee BM, Choi KH. Toxicometabolomics approach to urinary biomarkers for mercuric chloride (HgCl
2)-induced nephrotoxicity using proton nuclear magnetic resonance (
1H NMR) in rats. Toxicol Appl Pharmacol. 2010; 249(2):114–126. PMID:
20804780.
16. Kim KB, Yang JY, Kwack SJ, Kim HS, Ryu DH, Kim YJ, Bae JY, Lim DS, Choi SM, Kwon MJ, Bang DY, Lim SK, Kim YW, Hwang GS, Lee BM. Potential metabolomic biomarkers for evaluation of adriamycin efficacy using a urinary (1) H-NMR spectroscopy. J Appl Toxicol. 2012; 33(11):1251–1259.
17. Mendrick DL, Schnackenberg L. Genomic and metabolomic advances in the identification of disease and adverse event biomarkers. Biomark Med. 2009; 3(5):605–615. PMID:
20477528.

18. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004; 99(4):750–759. PMID:
15089911.

19. Walia R, Mahajan L, Steffen R. Recent advances in chronic constipation. Curr Opin Pediatr. 2009; 21(5):661–666. PMID:
19606041.

20. McCallum IJ, Ong S, Mercer-Jones M. Chronic constipation in adults. BMJ. 2009; 338:b831. PMID:
19304766.

21. Emmanuel AV, Tack J, Quigley EM, Talley NJ. Pharmacological management of constipation. Neurogastroenterol Motil. 2009; 21:41–54. PMID:
19824937.

22. Bharucha AE. Constipation. Best Pract Res Clin Gastroenterol. 2007; 21(4):709–731. PMID:
17643910.

23. Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation. Dis Colon Rectum. 1997; 40(3):273–279. PMID:
9118740.

24. Leung FW. Etiologic factors of chronic constipation: review of the scientific evidence. Dig Dis Sci. 2007; 52(2):313–316. PMID:
17219073.
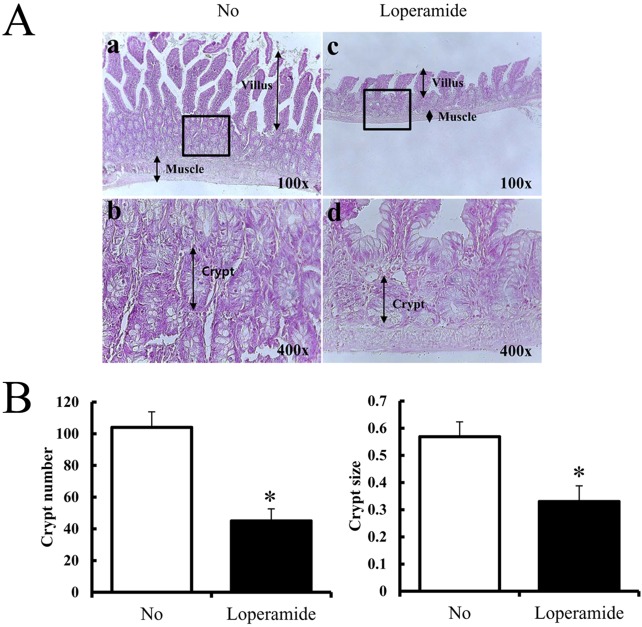
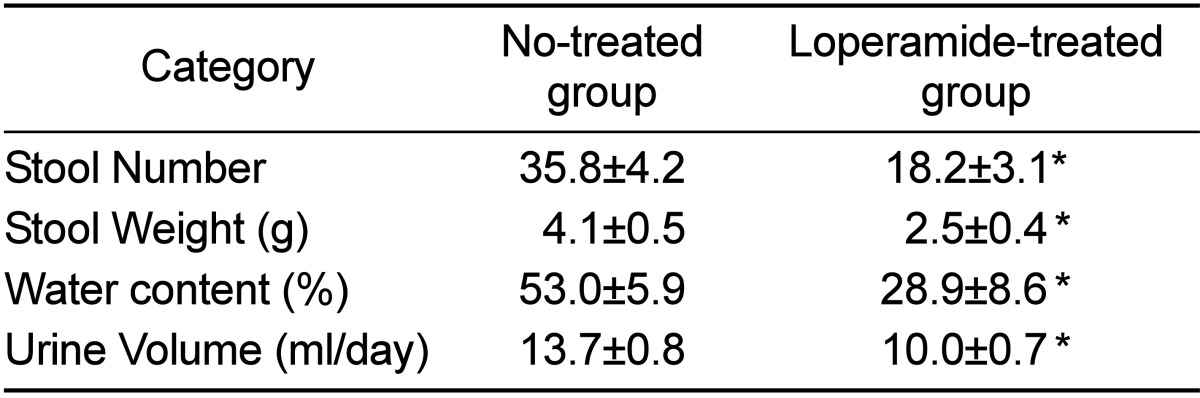
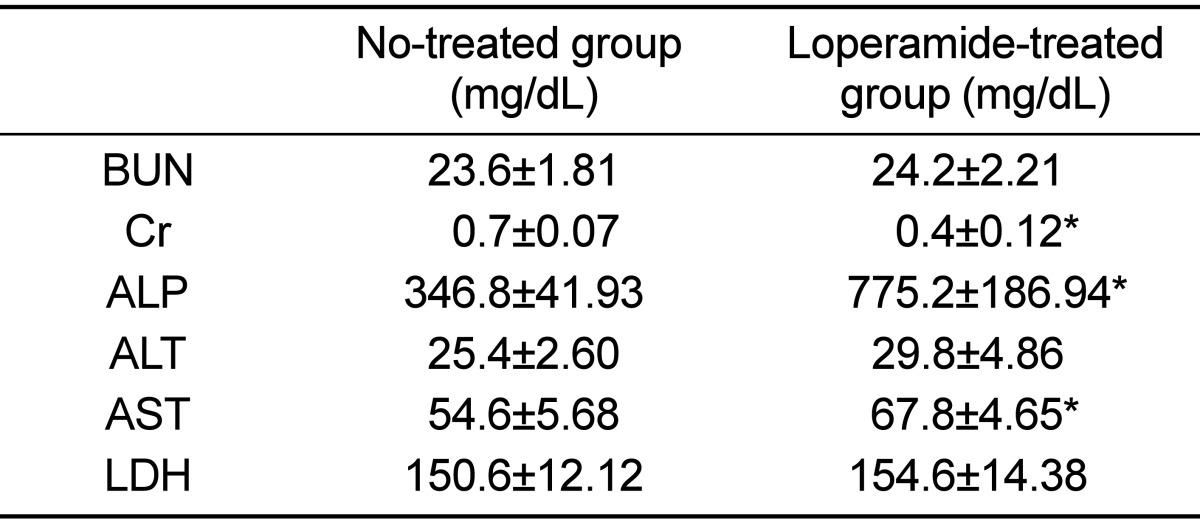
25. Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Decreased colonic mucus in rats with loperamide-induced constipation. Comp Biochem Physiol A Mol Integr Physiol. 2000; 126(2):203–212. PMID:
10936760.

26. Hou XY, Wang WL, Yong LI. DNA fingerprinting of intestinal flora in rats with slow transit constipation. J China Med Univ. 2013; 42:348–354.
27. Kakino M, Izuta H, Ito T, Tsuruma K, Araki Y, Shimazawa M, Oyama M, Iinuma M, Hara H. Agarwood induced laxative effects via acetylcholine receptors on loperamide-induced constipation in mice. Biosci Biotechnol Biochem. 2010; 74(8):1550–1555. PMID:
20699592.
28. Rodriguez L, Roberts LD, LaRosa J, Heinz N, Gerszten R, Nurko S, Goldstein AM. Relationship between postprandial metabolomics and colon motility in children with constipation. Neurogastroenterol Motil. 2013; 25(5):420–426. PMID:
23421516.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download