Abstract
Effects of FEMY-R7, composed of fucoidan and evening primrose extract, on the bacterial growth and intragastric infection of Helicobacter pylori as well as gastric secretion were investigated in comparison with a proton-pump inhibitor pantoprazole. For in vitro anti-bacterial activity test, H. pylori (1×108 CFU/mL) was incubated with a serially-diluted FEMY-R7 for 3 days. As a result, FEMY-R7 fully inhibited the bacterial growth at 100 µg/mL, which was determined to be a minimal inhibitory concentration. In addition, 6-hour incubation with H. pylori, FEMY-R7 inhibited urease activity in a concentration-dependent manner, showing a median inhibitory concentration of 1,500 µg/mL. In vivo elimination study, male C57BL/6 mice were infected with the bacteria by intragastric inoculation (5×109 CFU/mouse) 3 times at 2-day intervals, and simultaneously, orally treated twice a day with 10, 30 or 100 mg/kg FEMY-R7 for 7 days. In Campylobcter-like organism-detection test and bacterial identification, FEMY-R7 exerted a high bacteria-eliminating capacity at 30-100 mg/kg, comparably to 30 mg/kg pantoprazole. In contrast to a strong antacid activity of pantoprazole in a pylorus-ligation study, FEMY-R7 did not significantly affect gastric pH, free HCl, and total acidity, although it significantly decreased fluid volume at a low dose (10 mg/kg). The results indicate that FEMY-R7 eliminate H. pylori from gastric mucosa by directly killing the bacteria and preventing their adhesion and invasion, rather than by inhibiting gastric secretion or mucosal damage.
Go to : 
In peptic ulcers, there are mucosal injuries due to the gastric acid and pepsin, causing depletion of mucin secreted from mucus cells [1,2]. However, gastric erosions and ulcers are caused by various factors such as gastric over-secretion and retention, mucin layer-depleting agents, blood flow disturbances, and inflammation [1,2,3,4]. The ulcer-inducing agents include non-steroidal anti-inflammatory drugs (NSAIDs) that block production of prostaglandins (PGs), leading to mucin depletion and focal ischemia [5,6,7,8,9], alcohols [7,8,10], stresses [4,7,8], gastric retention [7,8], gastric hypermotility and acetic acid accumulation [8,11,12,13,14], and bacterial infection such as Helicobacter pylori [1,2,15,16,17].
H. pylori infection has been found in 70%, 50-60%, and 90% of gastric ulcer, gastritis, and duodenal ulcer patients, respectively. It is well known that H. pylori induce chronic active gastritis as a key factor for recurrence and aggravation of ulcers as well as development to gastric malignancies [18,19,20]. It is believed that H. pylori exacerbates erosions and ulcers by continuously stimulating gastric secretion and retention [21,22]. Accordingly, elimination of H. pylori is a key point for ulcer treatment in adults exhibiting a high incidence [1,2,15,16,17,23].
For the treatment of H. pylori-mediated gastritis and ulcers, triple therapies composed of proton-pump inhibitors (pantoprazole, omeprazole, lansoprazole, etc) and antibiotics (clarithromycin, metronidazole, amoxicillin, etc) have been recommended [2]. However, the antibiotics used for triple therapy are displaying a rapidly-increasing tolerance to H. pylori [24]. Therefore, natural products without tolerance during repeated treatments may contribute to the elimination of the bacteria.
Fucoidans, sulfate polysaccharide complex from seaweeds such as Laminaria japonica and Cladosiphon okamuranus, have been widely used as therapeutics in Oriental medicine. In previous studies, it have been demonstrated that fucoidan has anti-oxidative, anti-coagulative, anti-tumor, and anti-inflammatory activities [25,26]. Accordingly, the beneficial effects of fucoidan on inflammatory diseases, ischemia, immune dysfunction, and tumors are attracting investigators' attention [27,28]. Recently, fucoidan was demonstrated to inhibit the attachment of H. pylori to gastric cells in Mongolian gerbils and in humans [29,30].
It was reported that evening primrose tannins have anti-bacterial activity against H. pylori [31]. In addition, evening primrose extract was demonstrated to inhibit bacterial growth in vitro and blocks adhesion and colonization of H. pylori in the gastric walls [32].
On the basis of anti-H. pylori and anti-inflammatory activities, we investigated the effects of FEMY-R7, a combinational preparation of fucoidan and evening primrose extract, on the in vitro bacterial growth, in vivo bacterial infection, and gastric secretion in comparison with a proton-pump inhibitor pantoprazole.
FEMY-R7 containing fucoidan and evening primrose seed extract (1:1) was obtained from Misuba RTech Co. (Asan, Korea). Fucoidan was extracted with an acid-hot water extraction method at pH 2.0 and 60℃ for 2 hours from Laminaria japonica. The extraction supernatant was neutralized with 10 N NaOH and filtered. After precipitation with ethanol, the filtrate was centrifuged and dried. Evening primrose seeds were defatted and extracted with 60% ethanol. The extract was filtered, concentrated, and dried. The 1:1 (v/v) mixture of fucoidan and evening primrose seed extract, named FEMY-R7, was stored at 2℃ until use.
H. pylori SS1 (ATCC49503) was obtained from American Type Culture Collection (Manassas, VA, USA), and cultured on brain heart infusion (BHI) broth in an anaerobic chamber with 10% CO2, 5% O2, and 85% N2 at 37℃ with enough humidity
The inhibitory capacity of FEMY-R7 against the growth of H. pylori was assessed using agar-dilution method [33]. In brief, serially-diluted FEMY-R7 (10-100 µg/mL) was added to Mueller Hinton agar containing 10% fetal bovine serum (FBS), and H. pylori was inoculated on the agar. After 3-day incubation at 37℃, the FEMY-R7 concentration fully inhibiting the bacterial growth was determined to be minimal inhibitory concentration (MIC).
In order to assess in vitro urease inhibition, H. pylori (1×108 CFU/mL) were incubated with FEMY-R7 (10-2,000 µg/mL) at 37℃ under stirring at 50 rpm for 6 hours, and then 50 µL of urea base (2% urea and 0.03% phenol red) was added and allowed to react for 30 min. Urease activity was quantified by measuring the optical density at 550 nm, and was presented as median inhibitory concentration (IC50).
Male C57BL/6 mice (body weights 25-27 g) and male SD rats (200-220 g) were procured from Daehan Biolink (Eumseong, Korea), and housed in a room with constant environmental conditions (23±2℃; 55±10% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). Pellet feed and purified water were available ad libitum. All the animal experiments were conducted according to the Standard Operation Procedures (SOP), and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Korea. For biosafety, the investigators were fully protected with sterilized clothes, masks, and gloves on SOP.
After 12-hour fasting, the mice (n=10/group) were orally inoculated with H. pylori SS1 (5×109 CFU/1 mL/mouse) 3 times at 2-day intervals, and simultaneously, orally treated twice a day with 10, 30 or 100 mg/kg FEMY-R7 for 7 days.
Three hours after the final administration, the mice were sacrificed and their gastric mucosa was biopsied for the detection of H. pylori. The biopsy samples (3×3 cm) from gastric pylorus were minced, applied to Campylobacter-like organism (CLO)-detection kits (Kimberly-Clark, Roswell, GA, USA), and incubated at 35℃ for 24 hours to examine urease activity. The reaction (color change) was determined as negative for bright yellow, false (partially) positive for thick yellow, or positive for thick (dark) red [16,17,33].
The stomach of mice was removed and fixed in neutral formalin solution. Paraffin-embedded tissue slides were stained with Giemsa, and examined under a light microscope for the observation of bacteria and tissue injury in the gastric mucosa.
After 48-hour fasting, the rats were orally administrated FEMY-R7 (10, 30 or 100 mg/kg) or pantoprazole (30 mg/kg) in 1% carboxymethylcellulose (CMC), and their pylorus was ligated with a 4-0 silk after median incision of abdomen from xiphoid process under anesthesia with ether [34]. Six hours after pylorus ligation, the rats were sacrificed under deep anesthesia with ether, and their stomach was removed after clamping the esophagus. Gastric fluid was collected and centrifuged at 3,000 rpm for 10 min. The volume and pH of the fluid were measured. Into aliquots (500 µL) of the fluid, Töpfel reagent (1% dimethylaminoazobenzene) or 1% phenolphthalein (10 µL each) were added, and titrated with 0.1 N NaOH solution to measure free HCl and total acidity, respectively [34].
Data were expressed as the mean±SEM. Statistical analysis was performed using an analysis of variance (ANOVA) followed by the Dunnett's multiple-range test correction with the aid of SPSS for Windows v.10.0 (Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Go to : 
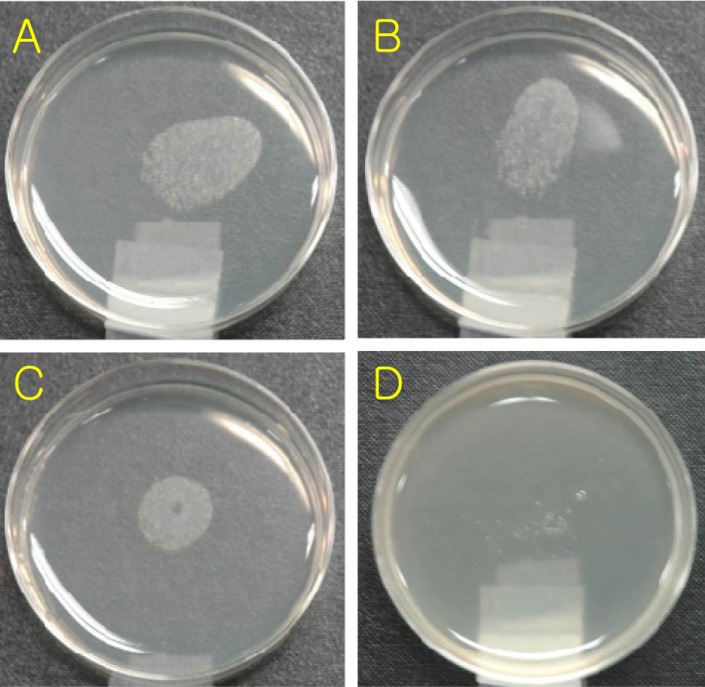
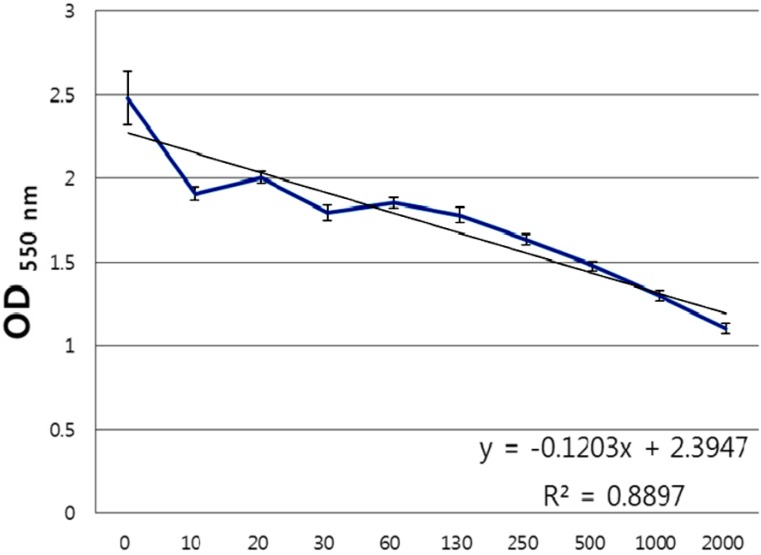
In vitro anti-bacterial test, 100 µg/mL FEMY-R7 completely inhibited the growth of H. pylori following 3-day incubation with the bacteria (1×108 CFU/mL) with a serially-diluted FEMY-R7, while 30 µg/mL FEMY-R7 slightly decrease the bacterial counts (Figure 1). Therefore, an MIC of FEMY-R7 was determined to be 100 µg/mL. In vitro urease-inhibition assay, FEMY-R7 inhibited urease activity in a dose-dependent manner (Figure 2), in which an IC50 of FEMY-R7 was determined to be 1,500 µg/mL.
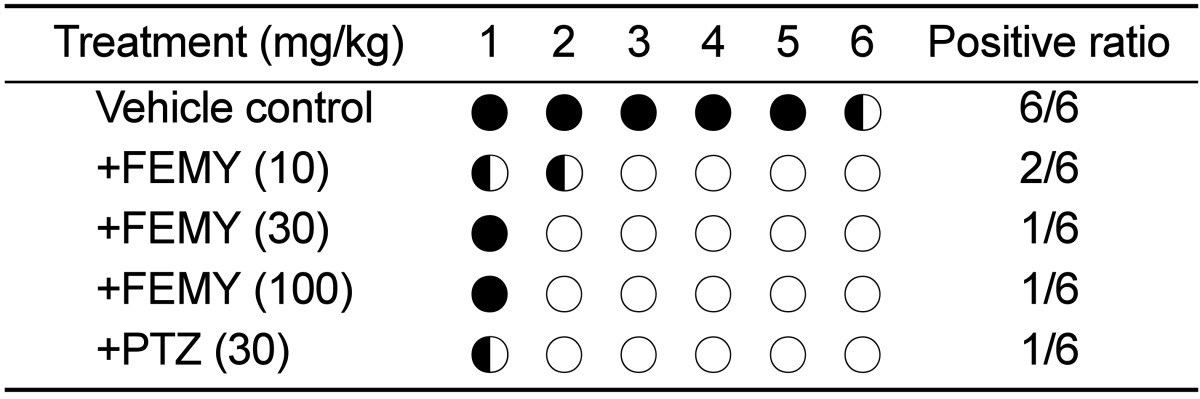
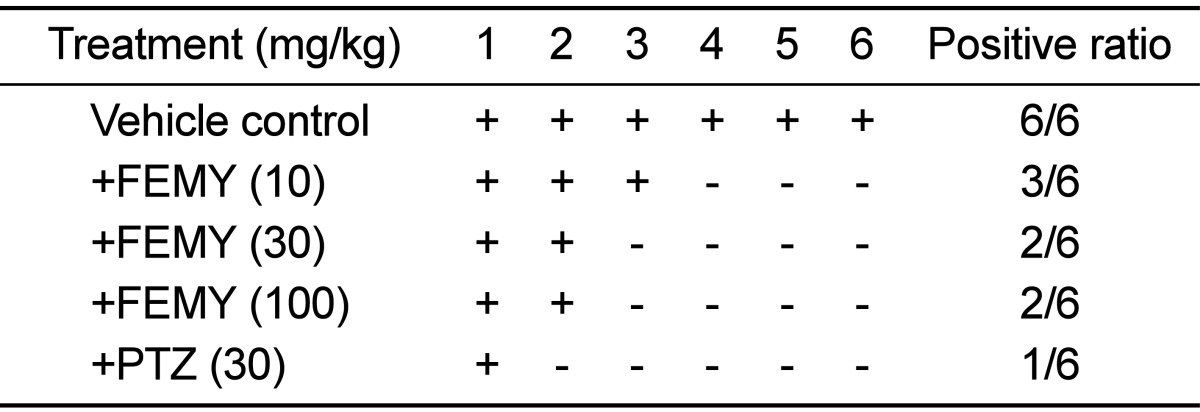
Repeated intragastric inoculation (5×109 CFU/mouse, 3 times) of H. pylori to C57BL/6 mice revealed positive reaction (red color) in CLO test. The mice orally treated with 10, 30 or 100 mg/kg FEMY-R7 twice a day for 7 days displayed positive reaction in 33.3% (2/6), 16.7% (1/6) and 16.7% (1/6) mice, respectively, in comparison with 100% (6/6) positivity in control (vehicle) group (Table 1). On the other hand, 16.7% (1/6) positive reaction was achieved following treatment with pantoprazole (30 mg/kg).
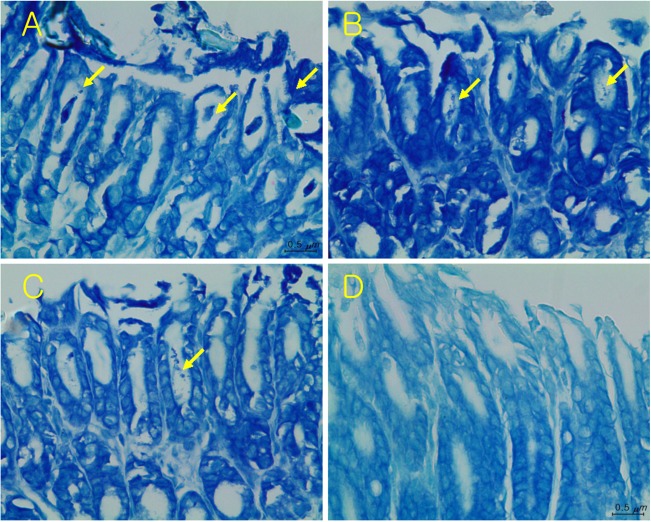
In the gastric mucosa of mice treated only with vehicle, several bacteria were detected in the area of mucosal injury (Figure 3A). In comparison, the detection ratio of bacteria and tissue injury were decreased and attenuated by treatment with FEMY-R7 and pantoprazole (Figure 3B-3D). Quantitatively, treatment with 10, 30 or 100 mg/kg FEMY-R7 displayed the presence of H. pylori in 50.0% (3/6), 33.3% (2/6) and 33.3% (2/6) mice, respectively, in comparison with 100% (6/6) detection in control group (Table 2). By comparison, pantoprazole (30 mg/kg), exhibiting 16.7% (1/6) positivity, was a little bit superior to FEMY-R7 (30-100mg/kg) in the elimination of the bacteria.
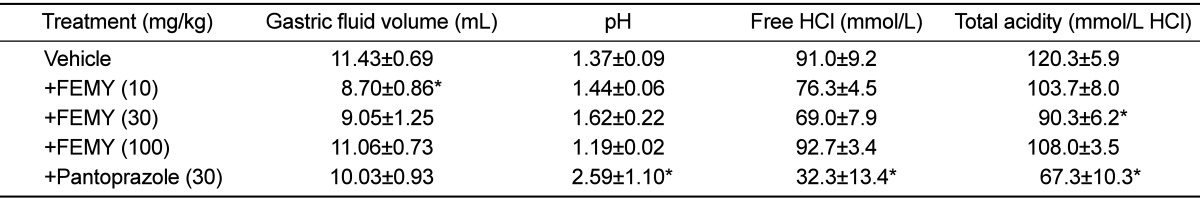
After 6-hour pylorus ligation, the gastric juice volume in control (vehicle) group was 11.43 mL/rat (Table 3). The gastric fluid volume was significantly decreased to 8.70 mL following treatment with a low dose (10 mg/kg) of FEMY-R7, while higher doses (30-100 mg/kg) of FEMY-R7 and pantoprazole (30mg/kg) did not significantly affect the gastric secretion. FEMY-R7 lowered total acidity and free HCl at only medium (30 mg/kg) and high (100 mg/kg) doses, respectively. By comparison, pantoprazole significantly increased gastric pH, and reduced both the free HCl and total acidity, indicative of a strong proton pump-inhibitory activity.
Go to : 
H. pylori is known to secrete urease to survive in acidic environment and invade gastric mucosa, causing gastritis, ulcers, and sometimes development of gastric cancer. In the present research, we demonstrated that FEMY-R7 has anti-bacterial activity against H. pylori with 100 µg/mL of MIC. Also, FEMY-R7 inhibited bacterial urease, in which the IC50 value was about 1,500 µg/mL. These results indicate that the urease-inhibitory activity of FEMY-R7 may not significantly contribute to anti-bacterial effect for inhibition of the bacterial growth.
In CLO test, treatment with FEMY-R7 for 7 days eliminated H. pylori from the stomach of mice, reaching efficacy over 50% at all doses used (10, 30, and 100 mg/kg). It is of interest to note that the effectiveness of FEMY-R7 at 30 or 100 mg/kg was comparable to that of 30 mg/kg pantoprazole, since it is well known that proton-pump inhibitors such as pantoprazole inhibit gastric secretion [35,36], protect parietal cells [7,37,38], and suppress H. pylori growth [39,40,41]. Compared with the results using CLO kits that detect urease activity, the bacterial cells were detected in more mice (Tables 1 and 2); i.e., the partially-positive stomachs in CLO test revealed H. pylori bacteria in Giemsa-stained mucosa (Figure 3). Therefore, it is assumed that some bacteria inactivated or killed not to produce urease may be stained by Giemsa.
The gastric secretion was inhibited at relatively-low doses (10-30 mg/kg) of FEMY-R7, whereas a high dose (100 mg/kg) of FEMY and pantoprazole (30 mg/kg) were ineffective. Notably, pH, free HCl, and total acidity of the gastric juice decreased at different dose ranges of FEMY-R7, but the effects of FEMY were negligible when compared to the excellent antacid activity of pantoprazole (Table 3). These results indicate that FEMY-R7 blocks the adhesion and invasion of H. pylori to mucosal walls by directly killing the bacteria as inferred from the relatively-low MIC. In spite of the high IC50 value in the inhibition of urease, it is believed that the enzyme-inhibitory activity of FEMY may also contributed to the bacterial clearance, because H. pylori is known to neutralize the gastric acid using the enzyme to survive there [42]. Such results and assumption were inferred from previous observations showed that fucoidan and evening primrose extract inhibited the adhesion and colonization of H. pylori to gastric cells [29,30,32].
In fact, in our follow-up studies to assess the effects of FEMY-R7 on the gastric mucosal injury, FEMY-R7 did not considerably attenuate the ulcer lesions induced by water-immersion restraint stress (WIRS), ethanol or indomethacin (an NSAID) (unpublished results). Such results support the conclusion that FEMY-R7 eliminates H. pylori from the gastric walls by killing the bacteria and inhibiting their invasion, but not by preserving or strengthening the mucosal layer including mucus content.
Effective treatment of H. pylori has been provided with triple therapies, for example, omeprazole + clarithromycin + amoxicillin or omeprazole +clarithromycin+metronidazole consisting of two antibiotics, which are still the most effective [2]. However, widespread use of antibiotics has often resulted in the development of resistance [24,43]. These problems indicate the need for the development of novel improved anti-bacterial agents with minimal or without resistance. In the present study, it was found that FEMY-R7 not only inhibited the growth of H. pylori
in vitro, but also eliminated the bacteria from the stomach walls in vivo. Notably, the effect of crude extract of FEMY-R7 was comparable to that of pantoprazole, a purified drug. The results indicate that FEMY-R7 could be a good candidate overcoming tolerance of antibiotics for the treatment of recurrent H. pylori infection.
Go to : 
References
1. Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996; 10(7):731–740.

2. Neal MJ. Medical Pharmacology at a Glance. 3rd ed. London: Blackwell Publishing Inc;2003. p. 30–31.
3. Isobe H, Okajima K, Harada N, Liu W, Okabe H. Activated protein C reduces stress-induced gastric mucosal injury in rats by inhibiting the endothelial cell injury. J Thromb Haemost. 2004; 2(2):313–320. PMID: 14995995.

4. Byun SK, Lee YE, Shin SH, Jang JY, Choi BI, Park DS, Jeon JH, Nahm SS, Hwang SY, Kim YB. The role of corticosteroids in stress-induced gastric ulceration in rats. Lab Anim Res. 2007; 23:127–131.
5. Slomiany BL, Piotrowski J, Slomiany A. Induction of tumor necrosis factor-alpha and apoptosis in gastric mucosal injury by indomethacin: effect of omeprazole and ebrotidine. Scand J Gastroenterol. 1997; 32(7):638–642. PMID: 9246701.
6. Filaretova L, Tanaka A, Miyazawa T, Kato S, Takeuchi K. Mechanisms by which endogenous glucocorticoid protects against indomethacin-induced gastric injury in rats. Am J Physiol Gastrointest Liver Physiol. 2002; 283(5):G1082–G1089.
7. Cao H, Wang MW, Jia JH, Wang QG, Cheng MS. Comparison of the effects of pantoprazole enantimers on gastric mucosal lesions and gastric epithelial cells in rats. J Health Sci. 2004; 50:1–8.
8. Rao ChV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, Pushpangadan P. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol. 2004; 91(2-3):243–249. PMID: 15120446.
9. Kim YR, Lee MR, Kim YH, Jang BJ, Park SC, Han SH, Kim BH, Ryoo ZY, Kim KS. Effect of Opuntiahumifusa extract on indomethacin-induced gastric ulcer in Sprague Dawley rat. Lab Anim Res. 2005; 21:375–578.
10. Raffin RP, Colomé LM, Schapoval EE, Jornada DS, Pohlmann AR, Guterres SS. Gastro-resistant microparticles containing sodium pantoprazole: stability and in vivo anti-ulcer activity. Open Drug Deliv J. 2007; 1:28–35.
11. Dias PC, Foglio MA, Possenti A, de Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethnopharmacol. 2000; 69(1):57–62. PMID: 10661884.
12. Cantarella G, Martinez G, Cutuli VM, Loreto C, D'Alcamo M, Prato A, Amico-Roxas M, Bernardini R, Clementi G. Adrenomedullin modulates COX-2 and HGF expression in reserpine-injuried gastric mucosa in the rat. Eur J Pharmacol. 2005; 518(2-3):221–226. PMID: 16081063.

13. Cantarella G1, Martinez G, Di Benedetto G, Loreto C, Musumeci G, Prato A, Lempereur L, Matera M, Amico-Roxas M, Bernardini R, Clementi G. Protective effects of amylin on reserpine-induced gastric damage in the rat. Pharmacol Res. 2007; 56(1):27–34. PMID: 17412609.

14. Işbil Büyükcoşkun N, Güleç G, Ozlük K. Protective effect of centrally-injected glucagon-like peptide-1 on reserpine-induced gastric mucosal lesions in rat: possible mechanisms. Turk J Gastroenterol. 2006; 17(1):1–6. PMID: 16830270.
15. Pope AJ, Toseland CD, Rushant B, Richardson S, McVey M, Hills J. Effect of potent urease inhibitor, fluorofamide, on Helicobacter sp. in vivo and in vitro. Dig Dis Sci. 1998; 43(1):109–119. PMID: 9508511.
16. Hahm KB, Kim DH, Lee KM, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Joo HJ, Cho YK, Nam KT, Cho SW. Effect of long-term administration of rebamipide on Helicobacter pylori infection in mice. Aliment Pharmacol Ther. 2003; 18(Suppl 1):24–38. PMID: 12925138.
17. Aristoteli LP, O'Rourke JL, Danon S, Larsson H, Mellgard B, Mitchell H, Lee A. Urea, fluorofamide, and omeprazole treatments alter helicobacter colonization in the mouse gastric mucosa. Helicobacter. 2006; 11(5):460–468. PMID: 16961809.

18. Coghlan JG, Gilligan D, Humphries H, McKenna D, Dooley C, Sweeney E, Keane C, O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers - a 12-month follow-up study. Lancet. 1987; 2(8568):1109–1111. PMID: 2890019.
19. Graham DY, Evans DG, Evans DJ Jr. Campylobacter pylori. The organism and its clinical relevance. J Clin Gastroenterol. 1989; 11(Suppl 1):S43–S48. PMID: 2809138.
20. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991; 338(8776):1175–1176. PMID: 1682595.
21. Cover TL, Blaser MJ. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992; 43:135–145. PMID: 1580578.
22. Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993; 61(5):1601–1610. PMID: 8478048.
23. Marshall BJ. Helicobacter pylori in peptic ulcer: have Koch's postulates been fulfilled? Ann Med. 1995; 27(5):565–568. PMID: 8541033.
24. Zhang Z, Liu ZQ, Zheng PY, Tang FA, Yang PC. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J Gastroenterol. 2010; 16(10):1279–1284. PMID: 20222174.
25. Feldman SC, Reynaldi S, Stortz CA, Cerezo AS, Damont EB. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine. 1999; 6(5):335–340. PMID: 11962540.
26. Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol. 2010; 46(1):6–12. PMID: 19883681.
27. Bojakowski K, Abramczyk P, Bojakowska M, Zwoliñska A, Przybylski J, Gaciong Z. Fucoidan improves the renal blood flow in the early stage of renal ischemia/reperfusion injury in the rat. J Physiol Pharmacol. 2001; 52(1):137–143. PMID: 11321507.
28. Li N, Zhang Q, Song J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem Toxicol. 2005; 43(3):421–426. PMID: 15680677.
29. Shibata H, Iimuro M, Uchiya N, Kawamori T, Nagaoka M, Ueyama S, Hashimoto S, Yokokura T, Sugimura T, Wakabayashi K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2003; 8(1):59–65. PMID: 12603617.
30. Shibata H, KimuraTakagi I, Nagaoka M, Hashimoto S, Sawada H, Ueyama S, Yokokura T. Inhibitory effect of Cladosiphon fucoidan on the adhesion of Helicobacter pylori to human gastric cells. J Nutr Sci Vitaminol (Tokyo). 1999; 45(3):325–336. PMID: 10524351.
31. Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, Hirai Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol Immunol. 2004; 48(4):251–261. PMID: 15107535.
32. Kamiya S, Osaki T, Yamaguci H, Shimada T, Okada T, Takahashi Y. Effect of evening primrose extract on growth, adhesion and colonization of Helicobacter pylori. Bact Adherence Bofilm. 2003; 17:16–19.
33. Yang YH, Park D, Yang G, Lee SH, Bae DK, Kyung J, Kim D, Choi EK, Son JC, Hwang SY, Kim YB. Anti-Helicobacter pylori effects of IgY from egg york of immunized hens. Lab Anim Res. 2012; 28(1):55–60. PMID: 22474475.
34. Jo IG, Park D, Kyung J, Kim D, Cai J, Kim J, Kwak TH, Yoo SK, Jeong HS, Kim YB. Inhibitory effects of a β-dunnione compound MB12662 on gastric secretion and ulcers. Lab Anim Res. 2013; 29(3):178–181. PMID: 24106514.

35. Takeuchi K, Konaka A, Nishijima M, Kato S, Yasuhiro T. Effects of pantoprazole, a novel H+/K+-ATPase inhibitor, on duodenal ulcerogenic and healing responses in rats: a comparative study with omeprazole and lansoprazole. J Gastroenterol Hepatol. 1999; 14(3):251–257. PMID: 10197495.
36. Cao H, Wang MW, Sun LX, Ikejima T, Hu ZQ, Zhao WH. Pharmacodynamic comparison of pantoprazole enantiomers: inhibition of acid-related lesions and acid secretion in rats and guinea-pigs. J Pharm Pharmacol. 2005; 57(7):923–927. PMID: 15969954.

37. Konturek SJ, Brzozowski T, Radecki T. Protective action of omeprazole, a benzimidazole derivative, on gastric mucosal damage by aspirin and ethanol in rats. Digestion. 1983; 27(3):159–164. PMID: 6354810.

38. Murakami I, Satoh H, Asano S, Maeda R. Role of capsaicin-sensitive sensory neurons and nitric oxide in the protective effect of lansoprazole, a proton pump inhibitor, on the gastric mucosa in rats. Jpn J Pharmacol. 1996; 72(2):137–147. PMID: 8912915.

39. Daw MA, Deegan P, Leen E, O'Moráin C. Short report: the effect of omeprazole on Helicobacter pylori and associated gastritis. Aliment Pharmacol Ther. 1991; 5(4):435–439. PMID: 1777552.
40. Iwahi T, Satoh H, Nakao M, Iwasaki T, Yamazaki T, Kubo K, Tamura T, Imada A. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother. 1991; 35(3):490–496. PMID: 2039199.
41. Hunt RH. Eradication of H. pylori infection. Am J Med. 1996; 100:42S–50S. PMID: 8644782.
42. Mobley HL, Hausinger RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989; 53(1):85–108. PMID: 2651866.

43. Brown ED, Wright GD. New targets and screening approaches in antimicrobial drug discovery. Chem Rev. 2005; 105(2):759–774. PMID: 15700964.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download