Abstract
We investigated the protective effects of pine bark extract (pycnogenol®, PYC) against cisplatin-induced hepatotoxicity and oxidative stress in rats. Twenty-four male rats were divided into the following four groups: (1) vehicle control, (2) cisplatin (7.5 mg/kg), (3) cisplatin & PYC 10 (10 mg/kg/day), and (4) cisplatin & PYC 20 (20 mg/kg/day). A single intraperitoneal injection of cisplatin induced hepatotoxicity, as evidenced by an increase in serum aminotransferase and histopathological alterations, including degeneration/necrosis of hepatocytes, vacuolation, and sinusoidal dilation. In addition, an increase in the malondialdehyde (MDA) concentration and a decrease in the reduced glutathione (GSH) content and catalase (CAT), superoxide dismutase (SOD), and glutathione S-transferase (GST) activities were observed in the cisplatin-treated rat hepatic tissues. In contrast, PYC treatment effectively prevented cisplatin-induced hepatotoxicity, including the elevation of aminotransferase and histopathological lesions, in a dosedependent manner. Moreover, PYC treatment also induced antioxidant activity by decreasing MDA level and increasing GSH content and SOD and GST activities in liver tissues. These results indicate that PYC has a protective effect against acute hepatotoxicity induced by cisplatin in rats, and that the protective effects of PYC may be due to inhibiting lipid peroxidation and increasing antioxidant activity.
Cisplatin (cis-diamminedichloroplatinum-II) is an effective chemotherapeutic agent to treat various solid tumors, including cancers of the ovary, cervix, colon, lung, and testis [1,2,3]. The chemotherapeutic mechanism of cisplatin involves cisplatin entering cells and its chloride ligands are replaced by water, and hydrated species that form react with nucleophilic sites on cellular macromolecules. The presence of cisplatin adducts in DNA sequences is thought to trigger cell cycle arrest and apoptosis [4,5]. Nevertheless, its clinical usefulness has frequently been limited by undesirable side effects on the kidneys, gastrointestinal tract, liver, peripheral nerves, inner ears, and testes [6,7,8]. Many studies on cisplatin toxicity have focused on well-known nephrotoxicity. However, some studies have shown that cisplatin also induces hepatotoxicity when it administered at a high dose and its mechanism may involve increased production of reactive oxygen species (ROS) and oxidative stress [9,10,11,12]. Therefore, it is essential to minimize potential side effect and preserved chemotherapeutic efficacy by co-administering an effective antioxidant agent to inhibit free radical generation. Antioxidants confer protection against cisplatin-induced oxidative stress in the liver [13,14].
Pine bark extract (Pycnogenol®, PYC) is a standardized proprietary mixture of bioflavonoids extracted from the bark of the French maritime pine (Pinus pinaster Aiton). It has been used in medically in Europe and North America for inflammatory diseases and wound healing among other applications [15]. Major constituents of PYC are polyphenols, flavonoids, and oligomeric procyanidins [16]. Polyphenols are comprised a wide range of natural plant substances and almost all exhibit marked antioxidant activity. PYC is a potent antioxidant and much of its pharmacologic activity comes from antioxidant activity, as it enhances synthesis of antioxidative enzymes and regenerate vitamins C and E, but also acts as a free-radical scavenger [17]. For this reason, the diverse beneficial effects of PYC have been claimed to protect against various degenerative conditions caused by oxidative stress [18,19].
Despite the favorable pharmacological properties of PYC, its protective capacity against hepatotoxicity and oxidative stress caused by cisplatin has not been explored previously. The present study investigated the protective effects of PYC on cisplatin-induced hepatotoxicity and oxidative damage in rats based on its strong antioxidant properties.
Twenty-four male Sprague-Dawley rats aged 9 weeks were obtained from a specific pathogen-free colony at Samtako Co. (Osan, Republic of Korea) and used after 1 week of quarantine and acclimation. Two animals per cage were housed in a room maintained at 23±3℃ and a relative humidity of 50±10% with artificial lighting from 08:00 to 20:00 and with 13-18 air changes per hr. Commercial rodent chow (Samyang Feed, Wonju, Republic of Korea) sterilized by radiation and sterilized tap water were available ad libitum. The Institutional Animal Care and Use Committee of Chonnam National University approved the protocols for the animal study (CNU IACUC-YB-2012-6), and the animals were cared for in accordance with the Guidelines for Animal Experiments of Chonnam National University.
Cisplatin (CAS No. 15663-27-1) was purchased from Sigma Aldrich Co. (St. Louis, MO, USA). PYC was purchased from Horphag Research Ltd. (Route de Belis, Le Sen, France). All other chemicals were of the highest grade commercially available. Test chemicals were dissolved in sterilized normal saline and were prepared immediately before treatment. The daily application volumes of cisplatin (2 mL/kg body weight) and PYC (10 mL/kg body weight) were calculated based on the most recently recorded body weight of the individual animal. PYC was gavaged to rats once daily for 10 days at dose levels of 10 and 20 mg/kg/day. The rats were given a single intraperitoneal dose of cisplatin (7.5 mg/kg) to induce liver injury 1 h after the PYC treatment on test day 5 [20]. All animals were sacrificed 5 days after administration of cisplatin (test day 10).
All animals were observed daily for clinical signs of toxicity and mortality throughout the study period. Abnormal signs were recorded individually by type, observation day, and time. The body weight of each rat was measured every other day.
All treated animals were euthanized with carbon dioxide for blood samples on the scheduled termination day (test day 10). Blood samples were drawn from the posterior vena cava, and serum samples were collected by centrifugation at 800×g for 10 min within 1 h after collection and stored at -80℃ before analysis. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured with an autoanalyzer (Dri-chem 4000i, Fujifilm Co., Tokyo, Japan). The absolute and relative (organ-to-body weight ratio) weights of the liver were measured.
A portion of the liver was dissected and fixed in 10% neutral buffered formalin solution for 2 weeks. The remaining liver portion was frozen quickly in dry ice and stored at -80℃ for biochemical analyses. The fixed tissues were processed routinely, embedded in paraffin, sectioned to 4 µm thickness, deparaffinized, and rehydrated using standard techniques. The extent of cisplatininduced liver injury and the protective effects of PYC were evaluated by assessing morphological changes in liver sections stained with hematoxylin and eosin. All observations were made manually with a light microscope with ×5, ×10, ×20, and ×40 objective lenses, as well as a ×100 oil immersion lens in a totally blinded manner. The following variables were used to assess histological changes in the liver: (1) hepatocyte degeneration/necrosis; (2) vacuolation; and (3) sinusoidal dilation.
A portion of frozen liver was homogenized in a glass-Teflon homogenizer with 50 mM phosphate buffer (pH 7.4) to obtain a 1:9 (w/v) whole homogenate. The homogenates were centrifuged at 11,000×g for 15 min at 4℃ to remove cell debris, and the supernatant was used to measure malondialdehyde (MDA) and GSH concentrations. MDA concentrations were assayed by monitoring the formation of thiobarbituric acid reactive substance using the method of Berton et al. (1998) [23]. GSH content was measured by the method of Moron et al. (1979) [24]. Antioxidant enzyme activities, including catalase (CAT), superoxide dismutase (SOD), and glutathione S-transferase (GST) were determined using commercial assay kits (Cayman Chemical, Ann Arbor, MI, USA). Total protein content was determined by the method of Lowry et al. (1951) [25], using bovine serum albumin as the standard.
Data are expressed as mean±standard deviation, and all statistical comparisons were made by one-way analysis of variance followed by the Tukey-Kramer or Dunnett's multiple comparison tests. The data were analyzed with GraphPad InStat ver. 3.0 (GraphPad Software, Inc., La Jolla, CA, USA). Differences with a P-value 0.05 were considered statistically significant.
No treatment-related mortality was observed in animals treated with PYC and cisplatin during the study period (data not shown). However, the cisplatin group showed treatment-related clinical signs, including depression (n=1) and piloerection (n=4) 10 min after administering cisplatin. Loss of fur (n=2) was observed on day 2. In contrast, no clinical signs were detected in the cisplatin & PYC groups. A significant decrease in body weight was observed on day 8 and 10 in the cisplatin group, compared to those in the control group (data not shown). No significant differences in body weights were observed between the cisplatin group and cisplatin & PYC groups. In addition, no significant differences were observed in the absolute or relative liver weights between the groups (data not shown).
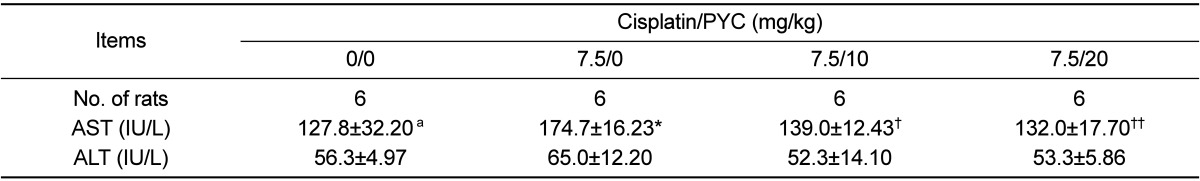
As shown in Table 1, serum AST level in the cisplatin group increased significantly compared to that in the control group. Although serum ALT level in the cisplatin group also increased slightly, no significant differences were observed between the cisplatin group and cisplatin & PYC groups. In contrast, AST levels in the cisplatin & PYC groups decreased significantly in a dosedependent manner compared to that in the cisplatin group.
The control group had livers with normal architecture (Figure 1A). However, liver tissue in the cisplatin group developed various histopathological changes, including degeneration/necrosis of hepatocytes, cytoplasmic vacuolation, and sinusoidal dilation (Figure 1B). Although some of these findings were also observed in the cisplatin & PYC groups, the incidence and severity of histopathological lesions were less than those of the cisplatin group (Figure 1C, D).
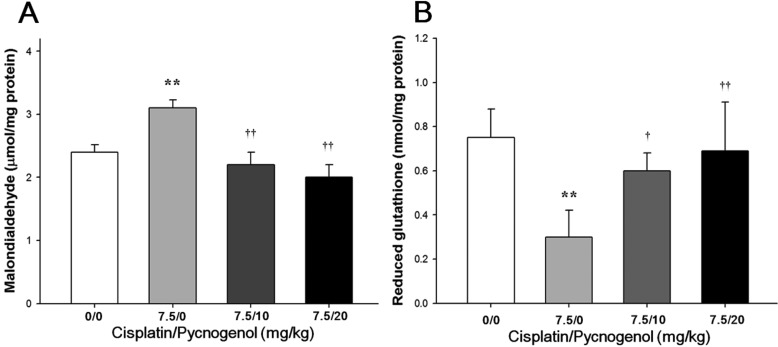
The MDA concentration and GSH activity in liver tissues are presented in Figure 2. The concentration of MDA, an end product of LPO, in rats treated with cisplatin increased significantly, whereas hepatic GSH content decreased significantly compared to those in the control group. However, hepatic MDA concentration decreased significantly in a dose-dependent manner in the cisplatin & PYC groups, compared to that in the cisplatin group. Hepatic GSH content also dose-dependently increased compared to that in the cisplatin group.
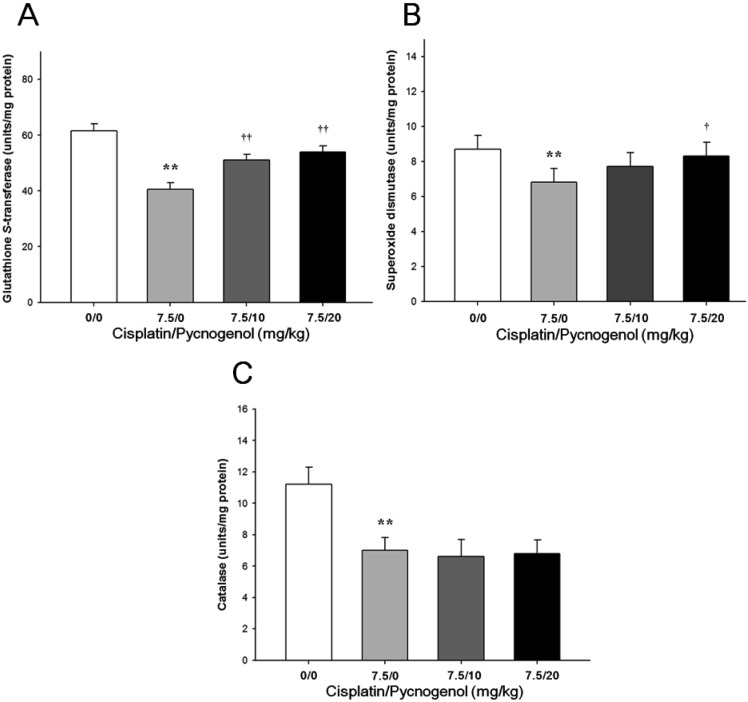
As presented in Figure 3, GST, CAT, and SOD activities in the cisplatin group decreased significantly compared to those in the control group. In contrast, GST and SOD activities in the cisplatin & PYC groups showed a dose-dependent increase compared to those in the cisplatin group. However, no significant differences were observed in CAT activities between the cisplatin and cisplatin & PYC groups.
Cisplatin is a highly active anti-neoplastic agent used to treat several types of cancer; however, its clinical utility is often limited by undesirable side effects on various organs including the liver [13]. Cisplatin interferes with the tissue antioxidant defense system and generates highly reactive oxygen free radicals that may be a predominant cause of its side effects [26]. One of the approaches to minimize cisplatin-induced hepatotoxicity is administration of protective antioxidant agents, such as selenium, fosfomycin, methionine, or taurine [27]. PYC is a potent antioxidant agent and affects both structural and functional characteristics of key enzymes and other cellular antioxidant networks and protects endogenous vitamin E and GSH [15,18]. Several studies have reported that PYC has protective effects against various pathological conditions and the side effects of other chemotherapeutic agents caused by oxidative stress [28,29,30]. Therefore, we hypothesized that PYC might be useful attenuating cisplatin-induced oxidative hepatic injury. Our results show that a single intraperitoneal injection of cisplatin resulted in severe hepatotoxicity and oxidative stress, as evidenced by increased serum aminotransferase levels and hepatic MDA content, histopathological alterations, and decreased GSH concentration and antioxidant enzymes activities. In contrast, PYC conferred a protective effect against hepatotoxicity and oxidative damage caused by cisplatin treatment in rats.
It has been well documented that cisplatin treatment for chemotherapy causes many clinical signs, particularly in the hair follicle, bone marrow, gastrointestinal tract, testis, and ovary, where cell differentiation occurs [31,32]. Treatment-related clinical signs, including depression, piloerection, and loss of fur were observed at high frequency in the cisplatin group. However, these clinical signs were not found in the control and PYC-treated groups. These results suggest that PYC effectively protects against cisplatin-induced adverse effects.
Serum aminotransferase activities have long been considered sensitive indicators of hepatic injury because injury to hepatocytes alters their transport function and membrane permeability causing leakage of enzymes from the cells [33]. In this study, a single intraperitoneal injection of cisplatin caused a significant increase in serum AST level and a slight increase in serum ALT level, suggesting the acute hepatotoxicity caused by cisplatin. These alterations correlated well with histopathological findings such as an increase in the incidence of hepatocellular degeneration/necrosis, vacuolation, and sinusoidal dilation. These results are in accordance with those of previous studies [34]. However, the groups treated with PYC were effectively protected from the cisplatin-induced elevation in serum AST and ALT levels, suggesting that PYC has hepatoprotective effects against acute toxicity induced by cisplatin. This finding was also confirmed by the histopathological examination results, as evidenced by a decrease in the incidence and severity of hepatic histopathological lesions caused by cisplatin.
Oxidative stress is generally defined as excess formation and/or insufficient removal of highly reactive molecules, such as reactive oxygen species (ROS) and reactive nitrogen species [35]. LPO is one of the principal causes of liver damage and its end product, MDA, is a major reactive aldehyde used as an oxidative stress marker [36]. GSH acts as a non-enzymatic antioxidant in conjugation with various enzymatic processes that reduce H2O2 and hydroperoxides by direct interaction of the -SH group with ROS [37]. The major antioxidant enzyme CAT is a primary H2O2 scavenger providing cellular defense against ROS [38]. Decreased CAT activity indicates reduced conversion of the superoxide radical to H2O2 by SOD. In this study, a single intraperitoneal injection of cisplatin increased hepatic MDA concentration and decreased GSH content and GST, SOD, and CAT activities, indicating that oxidative stress is associated with cisplatin-induced hepatotoxicity. In contrast, PYC attenuated the cisplatin-induced elevation in hepatic MDA concentration and improved cisplatininduced suppression of GSH content. These observations indicate that PYC effectively inhibited hepatic LPO and prevented depletion of GSH content induced by cisplatin. These actions were associated with GST, SOD, CAT activities in hepatic tissue. Those antioxidant enzymes activities improved in the PYC groups in principle. Although CAT activities in the PYC groups did not significantly improve compared to those in the cisplatin group, taken all our results together, these apparent ameliorative effects may be due to the ability of PYC to inhibit LPO and enhance antioxidant enzymes activities.
In conclusion, PYC had a protective effect against acute hepatotoxicity induced by cisplatin administration to rats, and the hepatoprotective effects of PYC may be due to inhibiting lipid peroxidation and increasing antioxidant activities. Our results suggest that PYC may be a useful protective agent against various cisplatin-induced side effects including hepatic injury caused by oxidative stress.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009602), Rural Development Administration, Republic of Korea. The animal experiment was supported by the Animal Medical Institute of Chonnam National University.
References
1. Wiltshaw E, Kroner T. Phase two study of cis-dichlorodimmine-platinum (II) in advanced adenocarcinoma of the ovary. Cancer Treat Rep. 1976; 60:55–60. PMID: 1000519.
2. Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000; 57:1229–1235. PMID: 11028915.

3. Crown JP. The platinum agents: a role in breast cancer treatment? Semin Oncol. 2001; 28:28–37. PMID: 11301372.

4. Pinedo HM, Schornagel JH. Platinum and Other Metal Coordination Compounds in Cancer Chemotherapy. New York: Plenum;1996.
5. Yang D, Wang AH. Structural studies of interactions between anticancer platinum drugs and DNA. Prog Biophys Mol Biol. 1996; 66(1):81–111. PMID: 9107133.

6. Rybak LP, Husain K, Whitworth C, Somani SM. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system. Toxicol Sci. 1999; 47(2):195–202. PMID: 10220857.

7. Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope. 1999; 109(11):1740–1744. PMID: 10569399.
8. Teranishi M, Nakashima T, Wakabayashi T. Effects of alpha-tocopherol on cisplatin-induced ototoxicity in guinea pigs. Hear Res. 2001; 151:60–70.
9. Cavalli F, Tschopp L, Sonntag RW, Zimmermann A. Cisplatin-induced hepatic toxicity. Cancer Treat Rep. 1978; 62:2125–2126. PMID: 751721.
10. Pollera CF, Ameglio F, Nardi M, Vitelli G, Marolla P. Cisplatin-induced hepatic toxicity. J Clin Oncol. 1987; 5:318–319. PMID: 3806175.

11. Cersosimo RJ. Hepatotoxicity associated with cisplatin chemotherapy. Ann Pharmacother. 1993; 27(4):438–441. PMID: 8477119.

12. Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci. 2006; 89(2):515–523. PMID: 16251482.

13. Liu J, Liu Y, Habeebu SS, Klaassen CD. Metallothionein (MT)-null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol. 1998; 149(2):24–41. PMID: 9512723.

14. Naziroglu M, Karaoðlu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004; 195(2-3):221–230. PMID: 14751677.

15. Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic Biol Med. 1999; 27(5-6):704–724. PMID: 10490291.

16. Guo Q, Zhao B, Packer L. Electron spin resonance study of free radicals formed from a procyanidin-rich pine (Pinus maritima) bark extract, pycnogenol. Free Radic Biol Med. 1999; 27(11-12):1308–1312. PMID: 10641725.

17. Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002; 40(4):158–168. PMID: 11996210.

18. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003; 17(1):24–38. PMID: 12616644.

19. Yang YS, Ahn TH, Lee JC, Moon CJ, Kim SH, Jun W, Park SC, Kim HC, Kim JC. Protective effects of Pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats. Food Chem Toxicol. 2008; 46(1):380–387. PMID: 17900780.

20. Ateşşahin A, Karahan I, Türk G, Gür S, Yilmaz S, Ceribaşi AO. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol. 2006; 21(1):42–47. PMID: 15979841.

21. Kim SH, Lee IC, Lim JH, Moon C, Bae CS, Kim SH, Shin DH, Park SC, Kim HC, Kim JC. Protective effects of pine bark extract on developmental toxicity of cyclophosphamide in rats. Food Chem Toxicol. 2012; 50(2):109–115. PMID: 22036974.

22. Maritim A, Dene BA, Sanders RA, Watkins JB 3rd. Effects of pycnogenol treatment on oxidative stress in streptozotocininduced diabetic rats. J Biochem Mol Toxicol. 2003; 17(3):193–199. PMID: 12815616.

23. Berton TR, Conti CJ, Mitchell DL, Aldaz CM, Lubet RA, Fischer SM. The effect of vitamin E acetate on ultraviolet-induced mouse skin carcinogenesis. Mol Carcinog. 1998; 23(3):175–184. PMID: 9833778.

24. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979; 582(1):67–78. PMID: 760819.

25. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265–275. PMID: 14907713.

26. Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007; 401(1):1–11. PMID: 17150040.

27. Grimm T, Schäfer A, Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radic Biol Med. 2004; 36(6):811–822. PMID: 14990359.

28. Belcaro G, Cesarone MR, Genovesi D, Ledda A, Vinciguerra G, Ricci A, Pellegrini L, Gizzi G, Ippolito E, Dugall M, Cacchio M, Di Renzo A, Stuard S. Pycnogenol may alleviate adverse effects in oncologic treatment. Panminerva Med. 2008; 50(3):227–234. PMID: 18927527.
29. Skeel RT, Ganz PA. Handbook of Cancer Chemotherapy. 8th Ed. Philadelphia: Wolters Kluwer;1999.
30. Kim JC, Kim KH, Chung MK. Testicular cytotoxicity of DA-125, a new anthracycline anticancer agent, in rats. Reprod Toxicol. 1999; 13(5):391–397. PMID: 10560588.

31. Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res. 2008; 57(2):125–131. PMID: 18282716.

32. Devaraj S, Vega-López S, Kaul N, Schönlau F, Rohdewald P, Jialal I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids. 2002; 37(10):931–934. PMID: 12530550.

33. Zimmerman HJ, Seeff LB. Enzymes in hepatic disease: In Diagnostic Enzymology. Philadelphia: Lea and Febiger;1970.
34. Cersosimo RJ. Hepatotoxicity associated with cisplatin chemotherapy. Ann Pharmacother. 1993; 27(4):438–441. PMID: 8477119.

35. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39(1):44–84. PMID: 16978905.

36. Quiles JL, Huertas JR, Battino M, Mataix J, Ramírez-Tortosa MC. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002; 180(10):79–95. PMID: 12324201.

37. Kadiiska MB, Gladen BC, Baird DD, Dikalova AE, Sohal RS, Hatch GE, Jones DP, Mason RP, Barrett JC. Biomarkers of oxidative stress study: are plasma antioxidants markers of CCl(4) poisoning? Free Radic Biol Med. 2000; 28(6):838–845. PMID: 10802213.

38. Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004; 216(1-2):31–39. PMID: 15109742.

Figure 1
Representative photographs of liver sections treated with (A) vehicle, (B) cisplatin (7.5 mg/kg), (C) cisplatin & PYC 10 (10 mg/kg) and (D) cisplatin & PYC 20 (20 mg/kg). Liver from cisplatin-treated rats showing moderate degeneration/necrosis of hepatocytes around the central vein region (open arrow head), vacuolation (closed arrow), and sinusoidal dilation (closed arrow head). H&E stain. Bar=50 µm (×400).
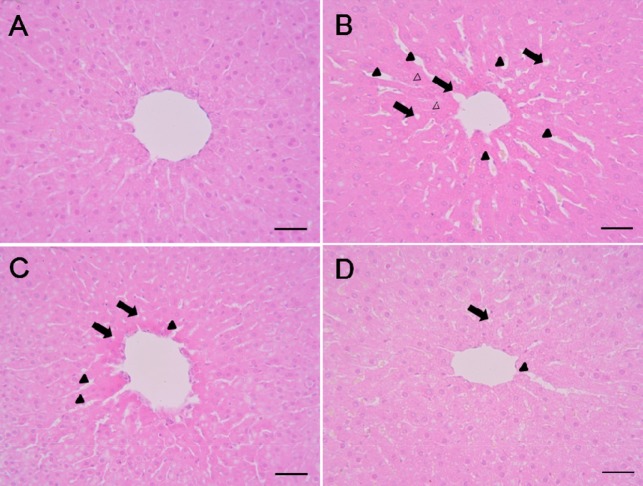
Figure 2
(A) Malondialdehyde (MDA) and (B) reduced glutathione (GSH) concentrations in the liver of male rats treated with cisplatin and/or PYC. Each bar represents the mean±SD (n=6). **P<0.01 versus the control group; ††P<0.01 versus the cisplatin group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download