1. Adjakly M, Ngollo M, Boiteux JP, Bignon YJ, Guy L, Bernard-Gallon D. Genistein and daidzein: different molecular effects on prostate cancer. Anticancer Res. 2013; 33(1):39–44. PMID:
23267126.
2. Adlercreutz H. Phytoestrogens: epidemiology and a possible role in cancer protection. Environ Health Perspect. 1995; 103(Suppl 7):103–112. PMID:
8593855.

3. Ahmad A, Biersack B, Li Y, Bao B, Kong D, Ali S, Banerjee S, Sarkar FH. Perspectives on the role of isoflavones in prostate cancer. AAPS J. 2013; 15(4):991–1000. PMID:
23824838.

4. Aidoo A, Bishop ME, Shelton SD, Lyn-Cook LE, Chen T, Manjanatha MG. Effects of daidzein, genistein, and 17beta-estradiol on 7,12-dimethylbenz[a]anthracene-induced mutagenicity and uterine dysplasia in ovariectomized rats. Nutr Cancer. 2005; 53(1):82–90. PMID:
16351510.
5. Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013; 698(1-3):31–38. PMID:
23178528.

6. Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004; 40(6):852–857. PMID:
15120041.
7. Bitto A, Polito F, Squadrito F, Marini H, D'Anna R, Irrera N, Minutoli L, Granese R, Altavilla D. Genistein aglycone: a dual mode of action anti-osteoporotic soy isoflavone rebalancing bone turnover towards bone formation. Curr Med Chem. 2010; 17(27):3007–3018. PMID:
20629630.

8. Cabanes A, Wang M, Olivo S, DeAssis S, Gustafsson JA, Khan G, Hilakivi-Clarke L. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004; 25(5):741–748. PMID:
14729590.

9. Carrillo-Infante C1, Abbadessa G, Bagella L, Giordano A. Viral infections as a cause of cancer (review). Int J Oncol. 2007; 30(6):1521–1528. PMID:
17487374.

10. Cheon DJ, Orsulic S. Mouse models of cancer. Annu Rev Pathol. 2011; 6:95–119. PMID:
20936938.

11. Constantinou AI, Lantvit D, Hawthorne M, Xu X, van Breemen RB, Pezzuto JM. Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr Cancer. 2001; 41(1-2):75–81. PMID:
12094632.

12. Croce CM. Oncogenes and cancer. N Engl J Med. 2008; 358(5):502–511. PMID:
18234754.

13. Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002; 60(3):205–211. PMID:
12031439.

14. Doyle A, McGarry MP, Lee NA, Lee JJ. The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 2012; 21(2):327–349. PMID:
21800101.

15. Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH, Wong WS. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am J Respir Crit Care Med. 2003; 167(2):185–192. PMID:
12406820.

16. El Touny LH, Banerjee PP. Akt GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis. 2007; 28(8):1710–1717. PMID:
17468512.
17. El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009; 69(8):3695–3703. PMID:
19351854.

18. Fan P, Fan S, Wang H, Mao J, Shi Y, Ibrahim MM, Ma W, Yu X, Hou Z, Wang B, Li L. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res Ther. 2013; 4(6):146. PMID:
24331293.

19. Fan Y, Wang C, Zhang Y, Hang P, Liu Y, Pan Z, Wang N, Du Z. Genistein ameliorates adverse cardiac effects induced by arsenic trioxide through preventing cardiomyocytes apoptosis. Cell Physiol Biochem. 2013; 31(1):80–91. PMID:
23363563.

20. Fritz WA, Coward L, Wang J, Lamartiniere CA. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998; 19(12):2151–2158. PMID:
9886571.

21. Gętek M, Czech N, Muc-Wierzgoñ M, Grochowska-Niedworok E, Kokot T, Nowakowska-Zajdel E. The active role of leguminous plant components in type 2 diabetes. Evid Based Complement Alternat Med. 2014; 293961. PMID:
24738003.

22. Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013; 4(2):200–212. PMID:
23160185.

23. Gingrich JR, Greenberg NM. A transgenic mouse prostate cancer model. Toxicol Pathol. 1996; 24(4):502–504. PMID:
8864193.

24. Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995; 92(8):3439–3443. PMID:
7724580.

25. Guo TL, Germolec DR, Zheng JF, Kooistra L, Auttachoat W, Smith MJ, White KL, Elmore SA. Genistein Protects Female Nonobese Diabetic Mice from Developing Type 1 Diabetes When Fed a Soy- and Alfalfa-free Diet. Toxicol Pathol. 2014.

26. Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992; 89(22):10578–10582. PMID:
1359541.

27. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100(1):57–70. PMID:
10647931.

28. Hilakivi-Clarke L, Onojafe I, Raygada M, Cho E, Skaar T, Russo I, Clarke R. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br J Cancer. 1999; 80(11):1682–1688. PMID:
10468283.

29. Hwang KA, Park MA, Kang NH, Yi BR, Hyun SH, Jeung EB, Choi KC. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 β-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor α and insulin-like growth factor-1 receptor signaling pathways. Toxicol Appl Pharmacol. 2013; 272(3):637–646. PMID:
23933164.
30. Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009; 61(5):598–606. PMID:
19838933.
31. Jin Z, MacDonald RS. Soy isoflavones increase latency of spontaneous mammary tumors in mice. J Nutr. 2002; 132(10):3186–3190. PMID:
12368416.

32. Kai K, Arima Y, Kamiya T, Saya H. Breast cancer stem cells. Breast Cancer. 2010; 17(2):80–85. PMID:
19806428.

33. Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003; 55(3):219–237. PMID:
12692788.

34. Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013; 22(3):305–310. PMID:
24078988.
35. Kulling SE, Honig DM, Metzler M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans
in vitro and
in vivo. J Agric Food Chem. 2001; 49(6):3024–3033. PMID:
11410004.
36. Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, Pelling JC, Bergan RC. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008; 68(6):2024–2032. PMID:
18339885.

37. Lamartiniere CA, Moore J, Holland M, Barnes S. Neonatal genistein chemoprevents mammary cancer. Proc Soc Exp Biol Med. 1995; 208(1):120–123. PMID:
7892285.

38. Lamartiniere CA, Moore JB, Brown NM, Thompson R, Hardin MJ, Barnes S. Genistein suppresses mammary cancer in rats. Carcinogenesis. 1995; 16(11):2833–2840. PMID:
7586206.

39. Lee JY, Kim HS, Song YS. Genistein as a Potential Anticancer Agent against Ovarian Cancer. J Tradit Complement Med. 2012; 2(2):96–104. PMID:
24716121.

40. Lee SH, Lee JH, Asahara T, Kim YS, Jeong HC, Ahn Y, Jung JS, Kwon SM. Genistein promotes endothelial colony-forming cell (ECFC) bioactivities and cardiac regeneration in myocardial infarction. PLoS One. 2014; 9(5):e96155. PMID:
24830850.

41. Li QS, Li CY, Li ZL, Zhu HL. Genistein and its synthetic analogs as anticancer agents. Anticancer Agents Med Chem. 2012; 12(3):271–281. PMID:
22043996.

42. Lian Z, Niwa K, Tagami K, Hashimoto M, Gao J, Yokoyama Y, Mori H, Tamaya T. Preventive effects of isoflavones, genistein and daidzein, on estradiol-17beta-related endometrial carcinogenesis in mice. Jpn J Cancer Res. 2001; 92(7):726–734. PMID:
11473722.
43. Liu MM, Huang Y, Wang J. Developing phytoestrogens for breast cancer prevention. Anticancer Agents Med Chem. 2012; 12(10):1306–1313. PMID:
22583409.
44. Mahn K, Borrás C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Viña J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure
in vivo. FASEB J. 2005; 19(12):1755–1757. PMID:
16107535.
45. Mai Z, Blackburn GL, Zhou JR. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog. 2007; 46(7):534–542. PMID:
17295235.

46. Marinković N, Pašalić D, Ferenčak G, Gršković B, Stavljenić Rukavina A. Dioxins and human toxicity. Arh Hig Rada Toksikol. 2010; 61(4):445–453. PMID:
21183436.

47. Mentor-Marcel R, Lamartiniere CA, Eltoum IA, Greenberg NM, Elgavish A. Dietary genistein improves survival and reduces expression of osteopontin in the prostate of transgenic mice with prostatic adenocarcinoma (TRAMP). J Nutr. 2005; 135(5):989–995. PMID:
15867270.

48. Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006; 98(18):1275–1284. PMID:
16985246.

49. Miao Q, Li JG, Miao S, Hu N, Zhang J, Zhang S, Xie YH, Wang JB, Wang SW. The bone-protective effect of genistein in the animal model of bilateral ovariectomy: roles of phytoestrogens and PTH/PTHR1 against post-menopausal osteoporosis. Int J Mol Sci. 2012; 13(1):56–70. PMID:
22312238.

50. Ming LG, Chen KM, Xian CJ. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J Cell Physiol. 2013; 228(3):513–521. PMID:
22777826.

51. Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y. Mechanism of 7,12-dimethylbenz[a]anthracene-induced immunotoxicity: role of metabolic activation at the target organ. Jpn J Pharmacol. 2001; 86(3):302–309. PMID:
11488430.

52. Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007; 2(2):247–250. PMID:
17406581.

53. Murrill WB, Brown NM, Zhang JX, Manzolillo PA, Barnes S, Lamartiniere CA. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996; 17(7):1451–1457. PMID:
8706248.
54. Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR, Wang Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One. 2011; 6(5):e20034. PMID:
21603581.

55. Nakamura H, Wang Y, Xue H, Romanish MT, Mager DL, Helgason CD, Wang Y. Genistein versus ICI 182, 780: an ally or enemy in metastatic progression of prostate cancer. Prostate. 2013; 73(16):1747–1760. PMID:
24038102.

56. Nguyen BT, Kararigas G, Jarry H. Dose-dependent effects of a genistein-enriched diet in the heart of ovariectomized mice. Genes Nutr. 2013; 8(4):383–390. PMID:
23108595.

57. Oates AJ, Barraclough R, Rudland PS. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumour model. Oncogene. 1996; 13(1):97–104. PMID:
8700559.
58. Onozawa M, Kawamori T, Baba M, Fukuda K, Toda T, Sato H, Ohtani M, Akaza H, Sugimura T, Wakabayashi K. Effects of a soybean isoflavone mixture on carcinogenesis in prostate and seminal vesicles of F344 rats. Jpn J Cancer Res. 1999; 90(4):393–398. PMID:
10363576.

59. Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010; 29(3):465–482. PMID:
20730632.

60. Pedraza-Farina LG. Mechanisms of oncogenic cooperation in cancer initiation and metastasis. Yale J Biol Med. 2006; 79(3-4):95–103. PMID:
17940619.
61. Raffoul JJ, Banerjee S, Che M, Knoll ZE, Doerge DR, Abrams J, Kucuk O, Sarkar FH, Hillman GG. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007; 120(11):2491–2498. PMID:
17304503.

62. Ruggeri BA, Camp F, Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol. 2014; 87(1):150–161. PMID:
23817077.

63. Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009; 48(4):213–220. PMID:
19234664.

64. Singh AV, Franke AA, Blackburn GL, Zhou JR. Soy phytochemicals prevent orthotopic growth and metastasis of bladder cancer in mice by alterations of cancer cell proliferation and apoptosis and tumor angiogenesis. Cancer Res. 2006; 66(3):1851–1858. PMID:
16452247.

65. Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005; 37(6):423–438. PMID:
16203615.

66. Song L, Liang X, Zhou Y. Estrogen-mimicking isoflavone genistein prevents bone loss in a rat model of obstructive sleep apnea-hypopnea syndrome. Int J Clin Exp Pathol. 2014; 7(4):1687–1694. PMID:
24817965.
67. Sutandyo N. Nutritional carcinogenesis. Acta Med Indones. 2010; 42(1):36–42. PMID:
20305331.
68. Tanaka T, Kohno H, Tanino M, Yanaida Y. Inhibitory effects of estrogenic compounds, 4-nonylphenol and genistein, on 7,12-dimethylbenz[a]anthracene-induced ovarian carcinogenesis in rats. Ecotoxicol Environ Saf. 2002; 52(1):38–45. PMID:
12051806.

69. Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of
in vitro, preclinical, and clinical studies. Nutr Rev. 2009; 67(7):398–415. PMID:
19566600.
70. Tham DM, Gardner CD, Haskell WL. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998; 83(7):2223–2235. PMID:
9661587.
71. Ueda M, Niho N, Imai T, Shibutani M, Mitsumori K, Matsui T, Hirose M. Lack of significant effects of genistein on the progression of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in ovariectomized Sprague-Dawley rats. Nutr Cancer. 2003; 47(2):141–147. PMID:
15087266.

72. Upadhyaya P, El-Bayoumy K. Effect of dietary soy protein isolate, genistein, and 1,4-phenylenebis(methylene)selenocyanate on DNA binding of 7,12-dimethylbenz[a]anthracene in mammary glands of CD rats. Oncol Rep. 1998; 5(6):1541–1545. PMID:
9769402.

73. Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, Colleoni M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011; 650(2-3):694–702. PMID:
21050844.

74. Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005; 65(8):3396–3403. PMID:
15833874.

75. Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A. Genistein as an anti-inflammatory agent. Inflamm Res. 2003; 52(8):341–346. PMID:
14504672.

76. Wang J, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. J Carcinog. 2007; 6:3. PMID:
17367528.

77. Wang X, Xin XY, Huang YH. [Regulative effect of genistein on xenografted tumor of ovarian carcinoma cell on nude mice]. Zhongguo Zhong Yao Za Zhi. 2006; 31(11):901–904. PMID:
17048629.
78. Wang Y, Raffoul JJ, Che M, Doerge DR, Joiner MC, Kucuk O, Sarkar FH, Hillman GG. Prostate cancer treatment is enhanced by genistein
in vitro and
in vivo in a syngeneic orthotopic tumor model. Radiat Res. 2006; 166(1 Pt 1):73–80. PMID:
16808622.
79. Wiegand H, Wagner AE, Boesch-Saadatmandi C, Kruse HP, Kulling S, Rimbach G. Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genomics Proteomics. 2009; 6(2):85–92. PMID:
19451092.
80. Wietrzyk J, Opolski A, Madej J, Radzikowski C. Antitumour and antimetastatic effect of genistein alone or combined with cyclophosphamide in mice transplanted with various tumours depends on the route of tumour transplantation. In Vivo. 2000; 14(2):357–362. PMID:
10836210.
81. Wu AH, Ziegler RG, Horn-Ross PL, Nomura AM, West DW, Kolonel LN, Rosenthal JF, Hoover RN, Pike MC. Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev. 1996; 5(11):901–906. PMID:
8922298.
82. Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998; 77(6):887–894. PMID:
9714059.

83. Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001; 61(Suppl 2):1–13. PMID:
11694782.

84. Zhang S, Wang Y, Chen Z, Kim S, Iqbal S, Chi A, Ritenour C, Wang YA, Kucuk O, Wu D. Genistein enhances the efficacy of cabazitaxel chemotherapy in metastatic castration-resistant prostate cancer cells. Prostate. 2013; 73(15):1681–1689. PMID:
23999913.

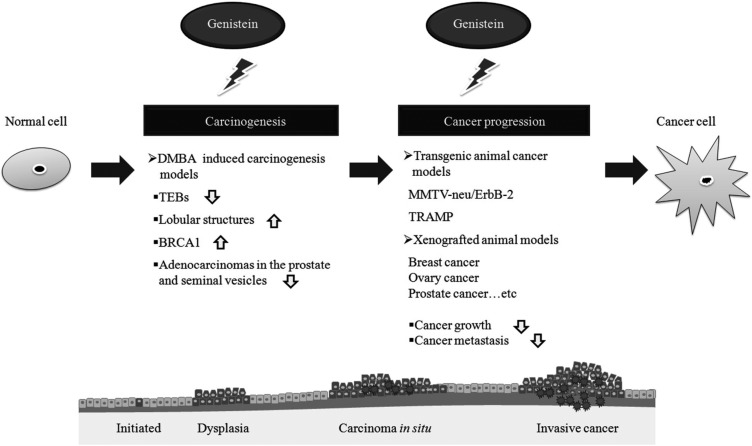




 PDF
PDF ePub
ePub Citation
Citation Print
Print


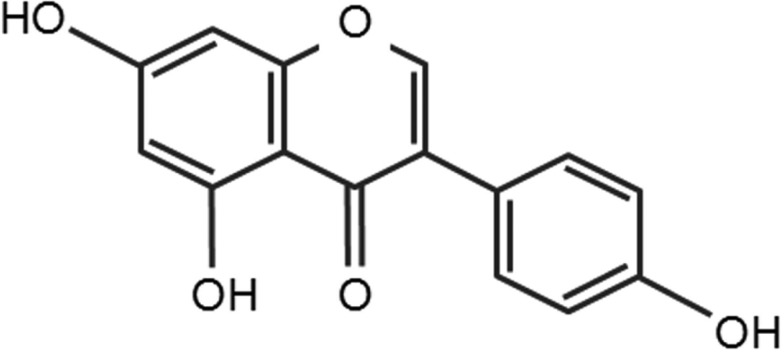
 XML Download
XML Download