Tympanic colic (TC) is characterized by the inability to expel gases produced during digestion by microbial fermentation, resulting in marked distension of the walls of cecum and colon. Colic is a major cause of illness and death in the horse [
1]. Gastric or intestinal tympany can be associated with inappropriate diet, overeating hay rich in clover or alfalfa, sudden dietary changes and gastrointestinal inflammation [
2,
3]. A change in the quality or the quantity of food, as well as a change in the schedule of feeding also results in an increased risk of colic [
4]. In some cases, such as grain overload, the proximate cause may be evident, but the underlying mechanism or physiologic problems often remain unknown [
5]. Acute distention of the gut wall and the consequent intestinal inflammation can lead to pronounced changes to the structure and physiology of the enteric nervous system (ENS). Considering the importance of the ENS in controlling secretion and motility in the gastrointestinal tract [
6,
7,
8], knowledge of the etiology of TC can contribute towards our understanding of the effects of similar digestive disorders in the ENS of other mammals, such as chronic constipation and bloat in ruminants or equine colic.
Various aspects of the anatomy, physiology and reproduction [
9,
10,
11,
12,
13] of
Chinchilla lanigera have been studied. Chinchillas are monogastric hindgut fermenting herbivores. Some authors compare them to horses ("miniature horses") in terms of gastrointestinal disease management [
14,
15]. The gastrointestinal anatomy of the chinchilla, such as a highly sacculated cecum and a large colon, predisposes it to TC [
9]. As chinchillas are more accessible to experimental approaches than equines or ruminants, we are proposing chinchillas as an experimental model for investigating the etiopathogeny of abdominal tympany.
Fifteen
Chinchilla lanigera from the Chillacenter Farm (Viamão, RS, Brazil) were used, (13 females and 2 males), aged 18 to 32 months and weighing between 400 to 700 g. The animals were kept individually in cages with free access to water and commercial food (Supra Chinchila, Alisul Alimentos SA, Brazil), a photoperiod regimen (12 h light/12 h dark) and controlled temperature (16-24℃). All animals were acclimated for three days before any experimental procedure (D0 at D3). They were then divided into
control (5 animals),
tympanic (5 animals) and
recovery (5 animals) groups. All animals were euthanatized by an overdose of ketamine and xylazine (Pfizer, Brazil). The experimental approach was approved by the local Committee for Ethics in Research (n° 2008148) and all animal procedures were in accordance with the Brazilian law (Federal Law n° 11.794/2008) on procedures for the scientific use of animals. The experimental design is depicted in
Figure 1.
 | Figure 1Time line of the experimental procedures. D0-D4: The acclimation period and first clinical examination. D4-D18: the tympanic colic period lasted from D4 to D18 and was divided in two shorter periods: disease induction (D4-D11) and disease maintenance (D11 to D18). The animals were examined and the mechanic hyper-sensitization was analyzed (von Frey test, D11 and D18). D18-D23: the recuperation period was divided in shorter periods: drug treatment (D18-D23: one daily injection of 1% Ketofen (2 mg/kg, IM) and post-treatment (D23-D38). On D23 and D38 the animals were examined and submitted to the von Frey test. 
|
The TC inducing protocol for chinchillas was adapted from the protocols of colic in equines [
16] and by analyzing the feeding practices that increase the risk of colic [
4]. Weiss et al. [
16] induces a laminitis secondary to intestinal impaction by grain overload. Among all the feeding patterns studied, changing the type of hay remains one of the most significant factors for colic development [
4]. As such, the TC protocol consisted of a colic-inducing diet (15% commercial food, 45% corn grains, 20% carrots, 5% sunflower seeds and 15% alfalfa daily). The chinchillas were fed at a fixed level (5% of body weight, BW; as-fed basis) that averaged 23.5 g/day, while water was freely accessible. The tympanic and recovery groups were fed with the colic-inducing diet daily for fifteen days (D3 to D18), while the control group was fed commercial food daily (5% of BW; as-fed basis) and 1.5% of BW of alfalfa (as-fed) once a week. This mixed diet was based on Wolf et al. [
17]. Alfalfa was cited as one of the most common cause of excessive gas production in equine and bovine. Many plant compounds, such as the triterpene saponins, have been suggested as contributing factors to the occurrence of equine colic or bovine bloat foam [
5,
18]. Vieira et al. [
19] analyzed 28 different Brazilian cultivars of alfalfa (
Medicago sativa L.) and found a maximum 1.78% of saponin in alfalfa. Regardless of the low or moderate level of saponins in alfalfa, the consumption of only small amounts of this leguminous is recommended for chinchillas. Once TC had been diagnosed (D18), the recovery group returned to the control feeding and were medicated with 1% ketoprofen (2 mg/kg Ketofen®, IM, Merial, Brazil) once a day, for five days (D18 to D23), when the clinical examinations were completed.
The clinical diagnosis was based on physical examination, using palpation, auscultation and abdominal percussion. The animals were examined on the first day (D0), after the acclimation period (D4), during the colic-inducing feed period for confirmation of disease onset (D11 and D18), and during the recuperation period of the recovery group (D23 and D38). The analysis of abdominal sensitization was tested with a mechanical force transducer (digital von Frey, Insight, Brazil) on the D11, D18, D23 and D38. Foot withdrawal frequencies in response to von Frey stimuli were measured and used to indicate abdominal mechanic sensitivity (
Figure 2). The Von Frey data were expressed as mean±SEM and repeated measures Analysis of Variance (ANOVA) was applied to the means to determine statistical differences between the experimental groups. Post-test comparisons were performed using the Bonferroni test. Differences between mean values were considered significant when
P<0.05. The tympanic group and three control animals were euthanized on D18, while the recovery group and two controls were euthanized when the anti-inflammatory treatment finished, on D38.
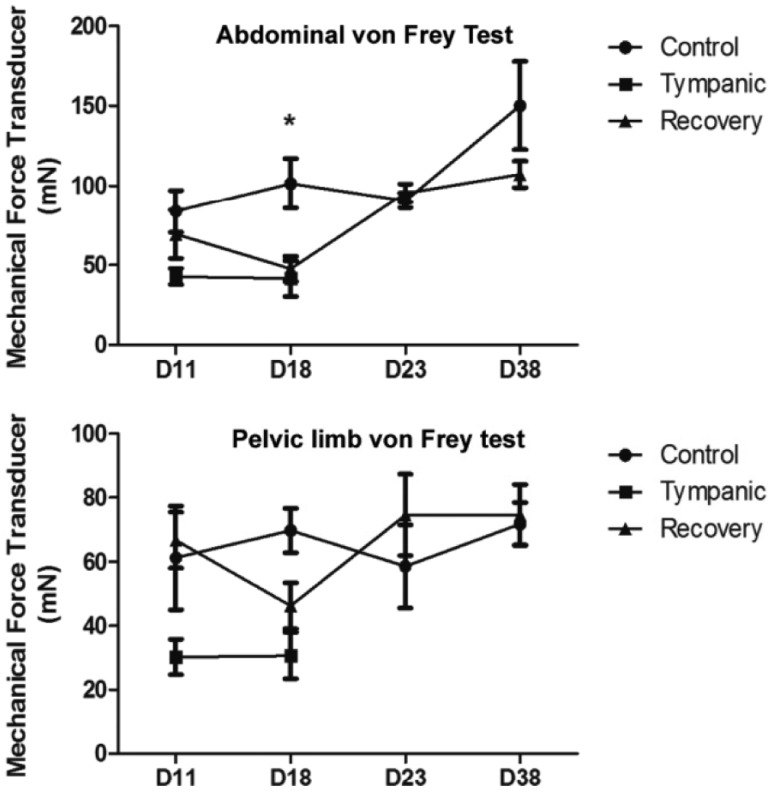 | Figure 2Response of abdominal sensitivity and foot withdrawal frequencies to a mechanical force transducer (von Frey test) in C. lanigera. In each animal, the mean force required, in milli-Newtons (mN), to evoke a response was calculated based on three measurements successively. The von Frey test was conducted on D11, D18, D23 and D38. Data presented as means±standard error (n=5/group). Repeated measures Analysis of Variance (ANOVA) and the Bonferroni test were applied. Asterisk indicates P<0.05, when the tympanic and recovery groups are compared with the control group. 
|
The animals did not present clinical alterations on D0 and D4. Seven days after the start of dietary change, D11, the animals from the tympanic group showed higher abdominal tension. The von Frey test revealed signs of abdominal sensitivity in the tympanic animals on D11, indicating the onset of the disease. These data were confirmed by observing foot withdrawal frequencies in response to von Frey stimuli (
Figure 2). On D18, these symptoms persisted in the tympanic animals, but the increase in abdominal percussion sounds was also evident, the abdomen was more swollen and the animals showed withdrawal behavior during palpation, indicating a possible hyperalgesic response. The von Frey test, performed on D18, revealed increased abdominal sensitivity in the tympanic animals (
Figure 2). After the anti-inflammatory treatment, on D23, the recovery animals did not show the symptoms observed on D18 and regained their appetite. There was a clear decrease of defecation from D11 to D20, after which control levels were re-established, according to the feeding behavior. The recovery group showed an abdominal sensitivity similar to that of the control group on D38, as measured by the von Frey test.
Modified anatomical aspects resulting from tympanic colic were confirmed by necropsy, before the large intestine was excised for other experiments (
Figure 3A-C). Histological sections of segments from the cecum and colon were stained with hematoxylin-eosin and used for histopathological analysis (
Figures 3E-G). Necropsy of the tympanic animals showed increased volume in the cecum, inter-cecal and ascending colon segments (
Figures 3C-D). Ischemic foci were found in the jejuno-ileal and ileocecal junctions, as well as in the ascending colon (
Figure 3D). Infarcted areas were also observed in the mesenteric vessels throughout the intestine (
Figure 3D). The transverse and descending colons showed fecal impaction while the rectum was empty. The cecal contents showed pH between 7.0 and 8.0, which supports a tympanic colic of the foamy or metabolic type. The tympanic animals had a 50% increase in the cecum volume (
Figure 3B), however, in the recovery animals these regions showed a volume similar to that of the control animals (
Figure 3A). No infarcted areas were found in the mesenteric blood vessels of the recovery animals (
Figure 3C).
 | Figure 3Ventral view of the abdominal cavity in necropsy observations (A-D) and histopathological alterations (E-G) occasioned by the experimental tympanic colic protocol in C. lanigera. A. Control animal. B. The tympanic animal presented an increase in volume in the cecum, inter-cecal and ascending colon segments. The abdominal viscera were displaced due to the increased volume of the cecum. C. In the recovery animal, after the drug treatment, the cecum volume returned to that observed in the control animals. D. Ischemic focuses and infarct in the mesenteric blood vessels (arrowheads) were observed in the tympanic animal, when compared with the control (A) and recovery (C) animals. E. Saccular cecum wall showed integrity in a control animal. F. Saccular cecum in the tympanic animal showed multiple macrophages, neutrophils and lymphocytes occupy the epithelial layer, and the lymphatic nodule extends to the lamina propria (right side). G. Tubular cecum showed an increase of inflammatory cells observed between the crypt and the lamina propria. Note the disruption of the muscularis mucosa. Hematoxylin and eosin stain (E-G). Are indicated: liver (l), stomach (s), duodenum (d), jejunum (j), saccular portion (sp) and tubular portion (t) of the cecum, ascending colon (aC), descending colon (dC), transverse colon (tC) and mesentery (m) and lymphatic nodule (ln). Bar: 2 cm (A-D) and 100 µm (E-G). 
|
Marked changes to the intestinal mucosa resulting from the tympany indicated a sub-acute inflammatory process of the cecum and ascending colon. The histopathological hallmark of these conditions was a dense leukocyte infiltrate mainly confined to the mucosa (
Figure 3F). The crypts of the cecum and ascending colon showed predominance of a denser inflammatory infiltrate, including macrophages, neutrophils and lymphocytes (
Figure 3G). The lamina propria of the ascending colon was more cellular than the transverse and descending colon and rectum. No signs of inflammation were observed in the large intestine of the control and recovery animals (
Figure 3E).
In the clinical analysis, macroscopic aspects of the intestine at necropsy and histopathological changes observed in the intestine of tympanic animals demonstrate that the designed protocol is suitable for inducing TC in
C. lanigera. According to Castro et al. [
9] the cecum of chinchillas has an average of 113.1 cm
3, and an increase of 50% of this organ was observed in the TC animals. The evident signs of mucosal inflammation found on D18 of the tympanic protocol had disappeared by D38, after the drug treatment and resumption of the control diet. Von Frey tests showed decreased abdominal sensitivity on D23, which became similar to the control data on D38 (
Figure 2). This suggests that the early events occurring during the acute abdomen episode (increase in cecal volume and mucosal inflammation) were responsible for the increased abdominal sensitivity on D18. Although cell death was not monitored in ischemic areas in
C. lanigera, cellular alterations were described in inflammatory bowel disease [
20,
21]. Fecal impaction and gas retention are consequences of a possible decrease in gut motility in tympanic chinchillas. Decreased propulsive motor activity was reported in guinea pig distal colon inflammation [
22,
23]. Eckert et al. [
24] observed neutropenia and lymphopenia in blood samples from a tympanic chinchilla. Increased levels of alkaline phosphatase (182 IU/L), creatine phosphokinase (1096 IU/L) and urea (51.60 mg/dL) were also found in these plasma samples. These data obtained from intestinal circulation indicate acute inflammation and damage to the GI tract and may reflect systemic evolution of TC. The infiltrating inflammatory cells reported in several experimental models of colitis in rodents evoke changes in the electrophysiological, neurochemical and morphological properties of the ENS and smooth muscle of the intestine [
25,
26,
27]. However, the specific effects of acute abdomen on the ENS remain largely unknown. Furthermore, the inflammatory responses to a given stimulus can vary among species [
28]. Experimental models of inflammation have included administration of chemical irritants such as acetic acid [
29], mustard oil or haptens such as trinitrobenzene sulfonic acid [
30,
31], infection with parasitic nematodes [
32,
33], addition of dextran sodium sulfate [
34] to drinking water, or treatment with a cell surface component of Gram-negative bacteria, lipopolysaccharide [
35]. The eighteen-day tympany experimental protocol did not cause a severe form of inflammation as did some of the above-mentioned substances, but instead represents an alteration that is more likely to occur due to the feeding mistakes occasionally observed in animal husbandry. These mistakes can result in unbalanced diets and digestive disorders in chinchillas or other small, domesticated mammals. The extent of the damage to intrinsic and extrinsic gut innervation evoked by experimental tympany is the object of our further studies.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download