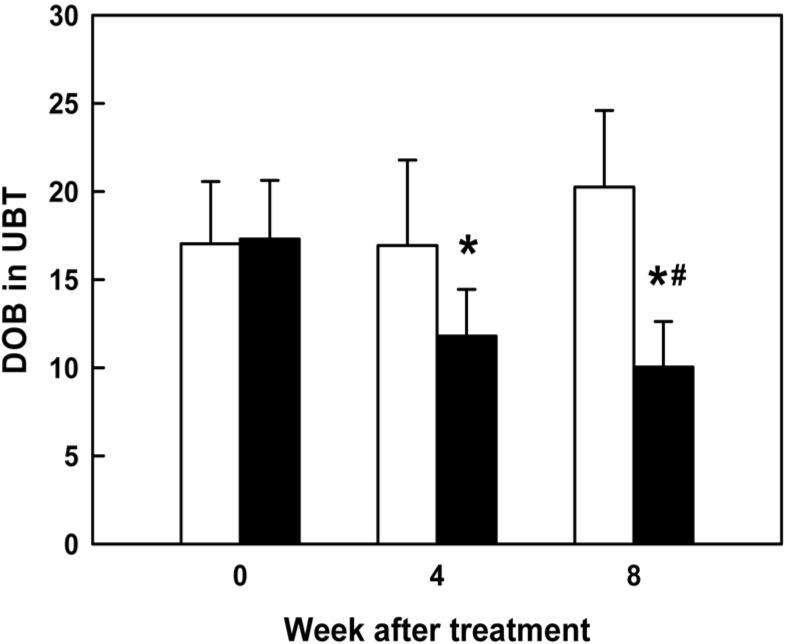
 | Figure 1Effect of FEMY-R7 (300 mg/day) on the Delta over baseline-value (DOB, ‰) in urea breath test (UBT) on the expiratory breath of patients infected with H. pylori. White: placebo, black: FEMY-R7. *Significantly different from before treatment (0 time). #Significantly different from placebo (8 week). |
Abstract
Helicobacter pylori-eliminating effects of FEMY-R7, composed of fucoidan and evening primrose extract, were investigated in mice and humans. Male C57BL/6 mice were infected with the bacteria by intragastric inoculation (1×109 CFU/mouse) 3 times at 2-day intervals, and simultaneously, orally treated twice a day with 10 or 100 mg/kg FEMY-R7 for 2 weeks. In Campylobcter-like organism-detection test, FEMY-R7 markedly reduced the urease-positive reactivity. In a clinical sudy, human subjects, confirmed to be infected with Helicobacter pylori, were orally administered twice a day with a capsule containing 150 mg FEMY-R7 for 8 weeks. FEMY-R7 significantly decreased both the Delta over baseline-value in urea breath test and the serum pepsinogens I and II levels. The results indicate that FEMY-R7 not only eliminates H. pylori from gastric mucosa of animals and humans, but also improves gastric function.
Go to : 
In peptic ulcers, gastric erosions and ulcers are caused by various factors such as gastric acid over-secretion and retention, mucin layer depletion, blood flow disturbances, and local inflammation [1,2,3,4]. There are many ulcer-inducing agents, including non-steroidal anti-inflammatory drugs (NSAID) [5,6,7,8,9], alcohols [7,8,10], stresses [4,7,8], gastric retention [7,8], gastric hypermotility and acetic acid accumulation [8,11,12,13,14], and bacterial infection such as Helicobacter pylori [1,2,15,16,17].
H. pylori infection is found in gastric ulcer (70%), gastritis (50-60%), and duodenal ulcer (90%) patients. It is well known that H. pylori is a key factor for chronic active gastritis as well as development to gastric cancers [18,19,20]. It is believed that H. pylori exacerbates erosions and ulcers via continuous stimulation of gastric secretion and retention [21,22]. Thus, eradication of H. pylori is a key point for ulcer treatment in adults exhibiting a high incidence [1,2,15,16,17,23].
For both the elimination of H. pylori and treatment of gastric ulcers, triple therapies containing proton-pump inhibitors (pantoprazole, omeprazole, lansoprazole, etc) and antibiotics (clarithromycin, metronidazole, amoxicillin, etc) have been recommended [2]. However, it is well known that the antibiotics used for triple therapy display a rapidly-increasing tolerance to H. pylori [24]. In spite of relatively-weak potency compared with antibiotics, therefore, natural products without tolerance during repeated administration may contribute to the eradication of the bacteria.
Fucoidan, sulfate polysaccharide complex from Laminaria japonica and Cladosiphon okamuranus, has been widely used in Oriental medicine. In previous studies, it has been demonstrated that fucoidan exerts anti-oxidative, anti-coagulative, and anti-inflammatory activities [25,26]. Accordingly, the beneficial effects of fucoidan on inflammatory diseases, ischemia, and immune dysfunction are attracting investigators' attention [27,28]. Recently, investigators showed that fucoidan inhibited the attachment of H. pylori to gastric cells in Mongolian gerbils and in humans [29,30]. Tannins from evening primrose have anti-bacterial activity against H. pylori, too [31]. Furthermore, evening primrose extract was found to inhibit bacterial growth in vitro and block adhesion and colonization of H. pylori in the gastric walls [32]. We also demonstrated that a combinational treatment with fucoidan and evening primrose extract killed H. pylori and eliminated the bacteria from the mouse stomachs in vitro and in vivo [33].
In the present study, we investigated the H. pylori-eliminating effects of FEMY-R7, a combinational preparation of fucoidan and evening primrose extract, in mice and in humans infected with H. pylori by confirming the presence of bacteria in Campylobcter-like organism (CLO)-detection test and urea breath test (UBT), respectively.
FEMY-R7 containing fucoidan and evening primrose seed extract (1:1) was obtained from Misuba RTech Co. (Asan, Korea). Fucoidan was extracted with an acid-hot water extraction method at pH 2.0 and 60℃ for 2 hours from Laminaria japonica [33]. The extraction supernatant was neutralized with 10 N NaOH and filtered. After precipitation with ethanol, the filtrate was centrifuged and dried. Evening primrose seeds were defatted and extracted with 60% ethanol. The extract was filtered, concentrated, and dried. The 1:1 (v/v) mixture of fucoidan (as L. japonica extract) and evening primrose seed extract, named FEMY-R7 [33], was stored at 2℃ until use. In Bio-LC analysis, FEMY-R7 was found to contain 7-15% fucose and 0.1-0.4% penta-O-galloyl-β-D-glucose.
Male C57BL/6 mice (body weights 25-27 g) were procured from Daehan Biolink (Eumseong, Korea), and housed in a room with constant environmental conditions (23±2℃; 55±10% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). Pellet feed and purified water were available ad libitum. All the animal experiments were conducted according to the Standard Operation Procedures (SOP), and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Korea (Approval No. CBNUR-284-11).
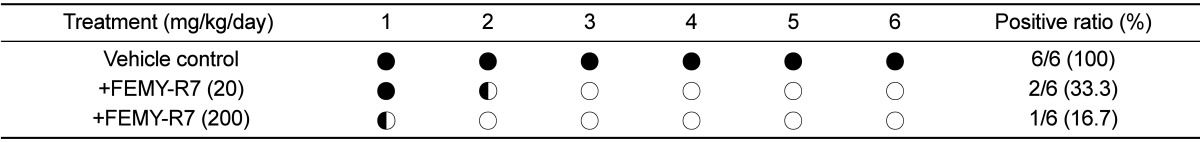
After 12-hour fasting, the mice (n=6/group) were orally inoculated with H. pylori SS1 (1×109 CFU/1 mL/mouse) 3 times at 2-day intervals, and simultaneously, orally treated twice a day with 10 or 100 mg/kg FEMY-R7 (20 or 200 mg/kg/day) for 2 weeks. Three hours after the final administration, the mice were sacrificed and their gastric mucosa was biopsied for the detection of H. pylori. The biopsy samples (3×3 cm) from gastric pylorus were minced, applied to CLO kits (Kimberly-Clark, Roswell, GA, USA), and incubated at 35℃ for 24 hours to examine urease activity. The reaction (color change) was determined as negative for bright yellow, false (partially) positive for thick yellow, or positive for thick (dark) red [16,17,33,34].
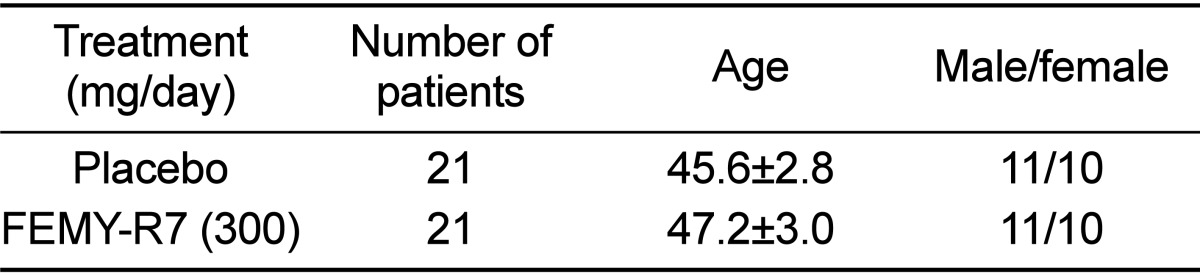
Among total of 60 patients (20-70 years old) who visited the Gastroenterology Department of the Catholic University Medical Center in Daegu, 42 patients (21 males, 21 females) were enrolled in this study (Table 1). Enrollment criteria included infection of H. pylori which were confirmed by gastric endoscopy and UBT. Exclusion criteria included patients with experience of H. pylori treatment, administration of NSAID or antibiotics within 4 weeks, heart failure, liver cirrhosis, severe cardiovascular, brain or renal diseases as well as pregnant or nursing women. The study protocol was approved by the Institutional review board (IRB) of the Catholic University Medical Center in Daegu (Approval No. CR-10-074-RES-01-R), and written informed consent was obtained from all patients.
We designed a randomized, double-blind, placebo-controlled clinical trial for eradication therapy of H. pylori. The patients in treatment group were given twice a day 1 FEMY-R7 capsule containing 75 mg fucoidan and 75mg evening primrose extract (300 mg/day) before a meal for 8 weeks. The patients in placebo control group were given only the capsules containing 150 mg microcrystalline cellulose. During the treatment period, the patients were requested to keep their own dietary lifestyle.
The presence of H. pylori in the stomach was detected by 13C-UBT [35,36]. Briefly, after 8-hour fasting, breath sample was collected in aluminized bag as the baseline value, followed by ingestion of 75 mg of 13C-urea powder (Helikit™; Isotechnika Inc, Edmonton, Canada) in 70 mL drinking water. Thirty min later, breath samples were collected, and analyzed by an isotope mass spectrometeter (Heliview; Medichems, Seoul, Korea) to measure the 13CO2/12CO2 ratio. A Delta over baseline-value (DOB) rised in the exhaled air by ≥4.0‰ was considered positive for H. pylori infection according to the manufacturer instruction and previous reports [35,36,37].
The fasting serum samples were collected from the patients, and both serum pepsinogen I and pepsinogen II levels were determined by a latex-enhanced turbidimetric immunoassay (HBi Co., Anyang, Korea) using an automated Toshiba-200FR system (Toshiba Medical Systems Co., Tokyo, Japan) [38,39,40].
Data were expressed as the mean±SEM. Statistical analysis was performed using an analysis of variance (ANOVA) followed by the Dunnett's multiple-range test correction with the aid of SPSS for Windows v.10.0 (Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Repeated intragastric inoculation (1×109 CFU/mouse, 3 times) of H. pylori to C57BL/6 mice revealed positive reaction (red color) in CLO test. The mice orally treated with 10 or 100 mg/kg FEMY-R7 twice a day for 2 weeks displayed positive reaction in 33.3% (2/6) and 16.7% (1/6) mice, respectively (Table 2). FEMY-R7 at 200 mg/kg/day near-fully eliminated the bacteria from the gastric wall, which was also confirmed in our previous study [33].
Based on the high effectiveness of FEMY-R7 in mice experimentally infected with H. pylori, we performed a clinical trial in naturally-infected patients. The initial conditions including age and gender were similar between placebo and treatment groups (Table 1). As analyzed by UBT, the BOD of patients treated with FEMY-R7 (300 mg/day) significantly decreased by 31.8% and 42% at 4 and 8 weeks, respectively (Figure 1), in comparison with no change in placebo group.
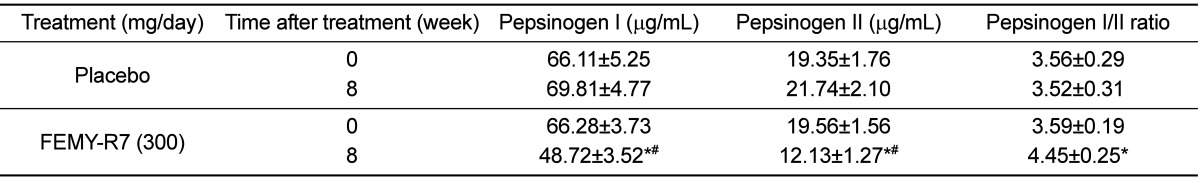
Notably, 8-week treatment with FEMY-R7 significantly decreased the serum pepsinogen I level, an atrophic gastritis marker (Table 3) [41,42]. Especially, FEMY-R7 more markedly reduced the pepsinogen II level, a surrogate marker of inflammatory response to H. pylori infection [38,39,40], leading to the increase in the pepsinogen I/II ratio. By comparison, both the serum pepsinogen I and II levels were not affected following intake of placebo capsules. On the other hand, FEMY-R7 did not affect the hematology [white blood cells, red blood cells (RBC), RBC indices, and platelets] and blood biochemistry related to liver function, kidney function, and energy and lipid metabolism (data not shown).
H. pylori is known to secrete urease to survive in acidic environment and to invade gastric mucosa, causing gastritis, ulcers, and sometimes development of gastric malignancies. In a previous [33] and the present investigations, we demonstrated that FEMY-R7 has an anti-bacterial activity against H. pylori. In CLO test, treatment with FEMY-R7 for 2 weeks eliminated H. pylori from the stomach of mice in a dose-dependent manner, displaying a near-full efficacy at 200 mg/kg/day. It is of interest to note that the H. pylori-eradicating effect of 200 mg/kg/day FEMY-R7 was comparable to that of 60 mg/kg/day pantoprazole, a well-known proton pump inhibitor [33]. More importantly, the effectiveness of FEMY-R7 was also confirmed in humans; i.e., daily intake of 300 mg FEMY-R7 for 8 weeks significantly lowered BOD in the expiratory breath.
In our previous study [33], it was found that FEMY-R7 eliminates H. pylori from the gastric walls by killing the bacteria and inhibiting their invasion, but not by preserving or strengthening the mucosal layer including mucus content. Actually, fucoidan and evening primrose extract inhibited the adhesion of H. pylori to gastric cells and their colonization there [29,30,32], which is a well-known action mechanism of proton-pump inhibitors that suppress H. pylori growth [43,44,45].
Notably, FEMY-R7 treatment decreased the blood levels of pepsinogens I and II. Since a part (approximately 1%) of the pepsinogens secreted from the stomach walls are released into blood, the levels of pepsinogens I and II and their ratio have been used as a surrogate marker of gastric secretory function [46,47,48]. Especially, the blood pepsinogen II level was proposed to be a potential marker of morphological change of the stomach during H. pylori-induced gastritis and tumor development [39]. Indeed, in previous studies [38,40], successful eradication therapy of H. pylori resulted in a decrease in the serum level of pepsinogen II, and ensuing an increase in the pepsinogen I/II ratio. In the present study, both the blood pepsinogens I and II decreased and the ratio of pepsinogen I/II increased following treatment with FEMY-R7, indicating that it improved the inflammatory response by eliminating H. pylori from the gastric mucosa.
Effective therapy of H. pylori has been provided with triple therapies (for example, omeprazole+clarithromycin+amoxicillin or omeprazole+clarithromycin+metronidazole) consisting of two antibiotics [2]. However, it has been well demonstrated that the widespread use of antibiotics led to a rapidly-increasing bacterial resistance [24,43]. Thus, there is a strong rationale for the development of novel anti-bacterial agents without resistance and adverse-effects. In the present study, it was found that FEMY-R7 not only eliminated H. pylori from the stomach walls of mice and humans, but also did not alter the hematological and blood biochemical parameters, related to tissue injuries, of human patients. Taken together, it is suggested that FEMY-R7, a combinational regimen composed of fucoidan and evening primrose extract, could be a promising candidate overcoming tolerance of antibiotics for the treatment of recurrent H. pylori infection.
References
1. Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996; 10(7):731–740. PMID: 8635690.

2. Neal MJ. Medical Pharmacology at a Glance. 3rd ed. London: Blackwell Publishing Inc;2003. p. 30–31.
3. Isobe H, Okajima K, Harada N, Liu W, Okabe H. Activated protein C reduces stress-induced gastric mucosal injury in rats by inhibiting the endothelial cell injury. J Thromb Haemost. 2004; 2(2):313–320. PMID: 14995995.

4. Byun SK, Lee YE, Shin SH, Jang JY, Choi BI, Park DS, Jeon JH, Nahm SS, Hwang SY, Kim YB. The role of corticosteroids in stress-induced gastric ulceration in rats. Lab Anim Res. 2007; 23(2):127–131.
5. Slomiany BL, Piotrowski J, Slomiany A. Induction of tumor necrosis factor-alpha and apoptosis in gastric mucosal injury by indomethacin: effect of omeprazole and ebrotidine. Scand J Gastroenterol. 1997; 32(7):638–642. PMID: 9246701.
6. Filaretova L, Tanaka A, Miyazawa T, Kato S, Takeuchi K. Mechanisms by which endogenous glucocorticoid protects against indomethacin-induced gastric injury in rats. Am J Physiol Gastrointest Liver Physiol. 2002; 283(5):G1082–G1089. PMID: 12381521.
7. Cao H, Wang MW, Jia JH, Wang QG, Cheng MS. Comparison of the effects of pantoprazole enantimers on gastric mucosal lesions and gastric epithelial cells in rats. J Health Sci. 2004; 50(1):1–8.
8. Rao ChV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, Pushpangadan P. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol. 2004; 91(2-3):243–249. PMID: 15120446.

9. Kim YR, Lee MR, Kim YH, Jang BJ, Park SC, Han SH, Kim BH, Ryoo ZY, Kim KS. Effect of Opuntiahumifusa extract on indomethacin-induced gastric ulcer in Sprague Dawley rat. Lab Anim Res. 2005; 21(4):375–578.
10. Raffin RP, Colomé LM, Schapoval EE, Jornada DS, Pohlmann AR, Guterres SS. Gastro-resistant microparticles containing sodium pantoprazole: stability and in vivo anti-ulcer activity. Open Drug Deliv J. 2007; 1:28–35.
11. Dias PC, Foglio MA, Possenti A, de Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethnopharmacol. 2000; 69(1):57–62. PMID: 10661884.
12. Cantarella G, Martinez G, Cutuli VM, Loreto C, D'Alcamo M, Prato A, Amico-Roxas M, Bernardini R, Clementi G. Adrenomedullin modulates COX-2 and HGF expression in reserpine-injuried gastric mucosa in the rat. Eur J Pharmacol. 2005; 518(2-3):221–226. PMID: 16081063.

13. Cantarella G, Martinez G, Di Benedetto G, Loreto C, Musumeci G, Prato A, Lempereur L, Matera M, Amico-Roxas M, Bernardini R, Clementi G. Protective effects of amylin on reserpine-induced gastric damage in the rat. Pharmacol Res. 2007; 56(1):27–34. PMID: 17412609.

14. Işbil Büyükcoşkun N, Güleç G, Ozlük K. Protective effect of centrally-injected glucagon-like peptide-1 on reserpine-induced gastric mucosal lesions in rat: possible mechanisms. Turk J Gastroenterol. 2006; 17(1):1–6. PMID: 16830270.
15. Pope AJ, Toseland CD, Rushant B, Richardson S, McVey M, Hills J. Effect of potent urease inhibitor, fluorofamide, on Helicobacter sp. in vivo and in vitro. Dig Dis Sci. 1998; 43(1):109–119. PMID: 9508511.
16. Hahm KB, Kim DH, Lee KM, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Joo HJ, Cho YK, Nam KT, Cho SW. Effect of long-term administration of rebamipide on Helicobacter pylori infection in mice. Aliment Pharmacol Ther. 2003; 18(Suppl 1):24–38. PMID: 12925138.

17. Aristoteli LP, O'Rourke JL, Danon S, Larsson H, Mellgard B, Mitchell H, Lee A. Urea, fluorofamide, and omeprazole treatments alter helicobacter colonization in the mouse gastric mucosa. Helicobacter. 2006; 11(5):460–468. PMID: 16961809.

18. Coghlan JG, Gilligan D, Humphries H, McKenna D, Dooley C, Sweeney E, Keane C, O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers--a 12-month follow-up study. Lancet. 1987; 2(8568):1109–1111. PMID: 2890019.
19. Graham DY, Evans DG, Evans DJ Jr. Campylobacter pylori. The organism and its clinical relevance. J Clin Gastroenterol. 1989; 11(Suppl 1):S43–S48. PMID: 2809138.
20. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991; 338(8776):1175–1176. PMID: 1682595.
21. Cover TL, Blaser MJ. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992; 43:135–145. PMID: 1580578.

22. Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993; 61(5):1601–1610. PMID: 8478048.

23. Marshall BJ. Helicobacter pylori in peptic ulcer: have Koch's postulates been fulfilled? Ann Med. 1995; 27(5):565–568. PMID: 8541033.
24. Zhang Z, Liu ZQ, Zheng PY, Tang FA, Yang PC. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J Gastroenterol. 2010; 16(10):1279–1284. PMID: 20222174.
25. Feldman SC, Reynaldi S, Stortz CA, Cerezo AS, Damont EB. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine. 1999; 6(5):335–340. PMID: 11962540.

26. Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol. 2010; 46(1):6–12. PMID: 19883681.

27. Bojakowski K, Abramczyk P, Bojakowska M, Zwoliñska A, Przybylski J, Gaciong Z. Fucoidan improves the renal blood flow in the early stage of renal ischemia/reperfusion injury in the rat. J Physiol Pharmacol. 2001; 52(1):137–143. PMID: 11321507.
28. Li N, Zhang Q, Song J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem Toxicol. 2005; 43(3):421–426. PMID: 15680677.

29. Shibata H, Iimuro M, Uchiya N, Kawamori T, Nagaoka M, Ueyama S, Hashimoto S, Yokokura T, Sugimura T, Wakabayashi K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2003; 8(1):59–65. PMID: 12603617.

30. Shibata H, KimuraTakagi I, Nagaoka M, Hashimoto S, Sawada H, Ueyama S, Yokokura T. Inhibitory effect of Cladosiphon fucoidan on the adhesion of Helicobacter pylori to human gastric cells. J Nutr Sci Vitaminol (Tokyo). 1999; 45(3):325–336. PMID: 10524351.

31. Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, Hirai Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol Immunol. 2004; 48(4):251–261. PMID: 15107535.
32. Kamiya S, Osaki T, Yamaguci H, Shimada T, Okada T, Takahashi Y. Effect of evening primrose extract on growth, adhesion and colonization of Helicobacter pylori. Bact Adherence Bofilm. 2003; 17:16–19.
33. Cai J, Kim TS, Jang JY, Kim J, Shin K, Lee SP, Choi EK, Kim SH, Park M, Kim JB, Kim YB. In vitro and in vivo anti-Helicobacter pylori activities of FEMY-R7 composed of fucoidan and evening primrose extract. Lab Anim Res. 2014; 30(1):28–34. PMID: 24707302.
34. Yang YH, Park D, Yang G, Lee SH, Bae DK, Kyung J, Kim D, Choi EK, Son JC, Hwang SY, Kim YB. Anti-Helicobacter pylori effects of IgY from egg york of immunized hens. Lab Anim Res. 2012; 28(1):55–60. PMID: 22474475.
35. Mattar R, Villares CA, Marostegam PF, Chaves CE, Pinto VB, Carrilho FJ. Low dose capsule based 13c-urea breath test compared with the conventional 13c-urea breath test and invasive tests. Arq Gastroenterol. 2014; 51(2):133–138. PMID: 25003266.

36. Oh DH, Lee DH, Kang KK, Park YS, Shin CM, Kim N, Yoon H, Hwang JH, Jeoung SH, Kim JW, Jang ES, Jung HC. The efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol. 2014; 29(6):1171–1176. PMID: 24955448.

37. Chobot A, Bak-Drabik K, Skała-Zamorowska E, Krzywicka A, Kwiecieñ J, Polañska J. Helicobacter pylori infection in type 1 diabetes children and adolescents using 13C urea breath test. Pol J Microbiol. 2014; 63(1):63–67. PMID: 25033664.

38. Choi HS, Lee SY, Kim JH, Sung IK, Park HS, Shim CS, Jin CJ. Combining the serum pepsinogen level and Helicobacter pylori antibody test for predicting the histology of gastric neoplasm. J Dig Dis. 2014; 15(6):293–298. PMID: 24602176.
39. Massarrat S, Haj-Sheykholeslami A, Mohamadkhani A, Zendehdel N, Aliasgari A, Rakhshani N, Stolte M, Shahidi SM. Pepsinogen II can be a potential surrogate marker of morphological changes in corpus before and after H. pylori eradication. Biomed Res Int. 2014; 2014:481607. PMID: 25028655.
40. Leja M, Lapina S, Polaka I, Rudzite D, Vilkoite I, Daugule I, Belkovets A, Pimanov S, Makarenko J, Tolmanis I, Lejnieks A, Boka V, Rumba-Rozenfelde I, Vikmanis U. Pepsinogen testing for evaluation of the success of Helicobacter pylori eradication at 4 weeks after completion of therapy. Medicina (Kaunas). 2014; 50(1):8–13. PMID: 25060199.

41. Mukoubayashi C, Yanaoka K, Ohata H, Arii K, Tamai H, Oka M, Ichinose M. Serum pepsinogen and gastric cancer screening. Intern Med. 2007; 46(6):261–266. PMID: 17379991.

42. Sipponen P, Härkönen M, Alanko A, Suovaniemi O. Diagnosis of atrophic gastritis from a serum sample. Clin Lab. 2002; 48(9-10):505–515. PMID: 12389711.
43. Daw MA, Deegan P, Leen E, O'Moráin C. Short report: the effect of omeprazole on Helicobacter pylori and associated gastritis. Aliment Pharmacol Ther. 1991; 5(4):435–439. PMID: 1777552.

44. Iwahi T, Satoh H, Nakao M, Iwasaki T, Yamazaki T, Kubo K, Tamura T, Imada A. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selective activity against Helicobacter pylori. Antimicrob Agents Chemother. 1991; 35(3):490–496. PMID: 2039199.

45. Hunt RH. Eradication of Helicobacter pylori infection. Am J Med. 1996; 100(5A):42S–51S. PMID: 8644782.

46. Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology. 1971; 61(2):185–188. PMID: 4935210.

47. Samloff IM, Liebman WM. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology. 1973; 65(1):36–42. PMID: 4124404.

48. Korstanje A, den Hartog G, Biemond I, Lamers CB. The serological gastric biopsy: a non-endoscopical diagnostic approach in management of the dyspeptic patient: significance for primary care based on a survey of the literature. Scand J Gastroenterol Suppl. 2002; 236:22–26. PMID: 12408500.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download