This article has been
cited by other articles in ScienceCentral.
Abstract
The present study investigated the potential subacute toxicity of 1,4-dichlorobutane by a 4-week repeated oral dose in Sprague-Dawley rats. The test article was administered once daily by gavage to male rats at dose levels of 0, 100, 300, and 1,000 mg/kg/day for 4 weeks. All rats were sacrificed at the end of the treatment period. During the test period, clinical signs, mortality, body weight, hematology, serum biochemistry, gross findings, and organ weight were examined. At 1,000 mg/kg/day, an increase in the clinical signs and weights of the liver and kidneys was observed in the male rats. Serum biochemical investigations revealed an increase in alanine aminotransferase, alkaline phosphatase, total cholesterol, total bilirubin, phospholipids, blood urea nitrogen, and gamma glutamyl transferase levels. There were no treatment-related adverse effects in the low and middle-dose groups. In the present experimental conditions, the target organs were determined to be liver and kidney. The no-observed-adverse-effect level was considered to be 300 mg/kg/day in rats.
Keywords: 1,4-Dichlorobutane, subacute toxicity, target organ, no-observed-adverse-effect level
Chloro-alkanes (C10-13), also called short chain chlorinated paraffins, are a complex mixture of closely related hydrocarbons with 10-13 carbon atoms arranged in chains and containing 50-70% chlorine by weight. Dichlorobutane is a chloroalkane with the molecular formula C
4H
8Cl
2, which is obtained by common chloroalkane synthetic methods. Dichlorobutane isomers are further utilized in various industrial and laboratory organic syntheses. Notably, 1,4-dichlorobutane (1,4-DCB) can be used as a precursor for nylon 6,6 or to produce tetrahydrofuran. 1,4-DCB is a colorless stable liquid with a mild pleasant odor. In addition, it is flammable and incompatible with strong bases and strong oxidizing agents. This chemical reacts vigorously with oxidizing materials [
1].
1,4-DCB is one of the high production volume chemicals with an annual production volumes >1 thousand metric tonnes (2.2 million pounds) in more than one economically developed country. The circulating amount of 1,4-DCB in Korea was 181,487 tonnes in 2007. As industrial workers may be exposed to 1,4-DCB at a high concentration during manufacture, it is important that the potential human health risks are assessed and that occupational exposures are managed accordingly. According to a single dose toxicity study, the oral LD
50 value for rats is 3,420 mg/kg. It was reported that 1,4-DCP does not induce micronuclei in bone marrow cells of ICR mice [
1]. However, no information is available on the potential subacute toxicity of 1,4-DCB after repeated oral exposure despite its widespread uses and exposure. Moreover, 1,4-DCB is not listed by AGGIH (American Conference of Industrial Hygienists), IARC (International Agency for Research on Cancer), and NTP (National Toxicology Program).
In the present study, we report the results of a 4-week repeated oral dose toxicity study in rats performed as part of a safety evaluation program for 1,4-DCB. Although the most likely route of exposure is inhalation, the oral route of exposure was selected for this study because there was a possibility of oral exposure, and oral dosing is more accurate to estimate the amount of test chemical ingested by an organism.
Materials and Methods
Animal husbandry and maintenance
Twenty-four male Sprague-Dawley rats aged 4 weeks were purchased from Orient Bio (Seoul, Korea) and were used after a 1 week quarantine and acclimatization period. Two animals per cage were housed in a room maintained at a temperature of 23±3℃ and a relative humidity of 50±10% with artificial lighting from 08:00 to 20:00 and with 13 to 18 air changes per hour. The animals were provided tap water sterilized by ultraviolet irradiation and commercial rodent chow (PMI Nutritional International Inc., Richmond, IN, USA) ad libitum. This experiment was conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, and all procedures were approved by the Institutional Animal Care and Use Committee, Korea Institute of Toxicology.
Test chemical and exposure
1,4-DCB was purchased from Sigma-Aldrich (St. Louis, MO, USA). The test chemical was dissolved in corn oil (Sigma-Aldrich) and dosing solutions were prepared daily before treatment. The daily application volume (4mL/kg body weight) of 1,4-DCB was calculated in advance based on the most recently recorded body weight of each individual animal. The test mixture was administered by gavage to male rats for 4 weeks. Vehicle control rats received an equivalent volume of corn oil alone.
Experimental groups and dose selection
Healthy male rats were assigned randomly to four experimental groups (n=6 per group): three treatment groups receiving 100, 300, and 1,000 mg/kg/day 1,4-DCB and a vehicle control group. Because the toxic potency of 1,4-DCB was expected to be low, a dose of 1,000mg/kg/day was selected as the standard toxicological limit dose that is recommended by OECD test guidelines. Doses of 300 and 100 mg/kg/day were selected as medium and low doses, respectively. In addition, a vehicle control group was added to determine the effects of the vehicle.
Clinical observation and mortality
All animals were observed twice daily (before and after exposure) throughout the study period for any clinical signs of toxicity and mortality.
Body weights
The body weight of each rat was measured at the beginning of exposure and once per week during exposure.
Hematology
The animals were fasted overnight before necropsy and blood collection. Blood samples were drawn from the posterior vena cava using a syringe with a 24-gauge needle under sodium isoflurane anesthesia. Blood samples were collected into CBC bottles containing EDTA-2K (Green Cross Medical Industry, Seoul, Korea), and analyzed within 20 minutes using an automatic hematology analyzer (Bayer ADVIA 120 Hematology Analyzer System, Leverkusen, Germany). The following parameters were determined: red blood cell count (RBC), hemoglobin concentration (HB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), reticulocytes (RET), platelet count (PLT), mean platelet volume (MPV), white blood cell count (WBC), and differential WBC count.
Serum biochemistry
Blood samples were centrifuged at 3,000 rpm for 10 minutes within 1 hour after collection. The sera were stored at -80℃ prior to analysis. The following serum biochemistry parameters were evaluated using an autoanalyzer (Toshiba 200FR NEO, Toshiba Co., Tokyo, Japan): aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CRTN), glucose, total cholesterol (T-CHO), total bilirubin (T-BIL), total protein (TP), albumin (ALB), creatine phosphokinase (CPK), triglycerides (TG), calcium, inorganic phosphorus (IP), phospholipid (PL), and gamma glutamyl transferase (GGT).
Gross findings
All surviving animals were anesthetized with isoflurane to collect blood samples at the end of the experiment. The rats were sacrificed by exsanguination from the abdominal aorta. Complete gross postmortem examinations were performed on all terminated animals.
Organ weights
Absolute and relative (organ-to-body weight ratios) weights of the following organs were measured: brain, liver, spleen, heart, seminal vesicles, prostate, kidneys, adrenal gland, testes, and epididymides.
Statistical analysis
Data are expressed as mean±standard deviation. Variance in the numerical data was checked by Bartlett's test [
2]. If the variance was homogeneous, the data were subjected to one-way analysis of variance (ANOVA) and, if not, they were analyzed by the Kruskal-Wallis non-parametric ANOVA [
3]. If either of the tests showed a significant difference among the groups, the data were analyzed by Dunnett's post-hoc test [
4]. Clinical signs and necropsy were represented as frequencies and were subjected to the Fisher's exact probability test [
5] when necessary. The statistical analysis was performed by comparing the treatment groups with the control group using the Path/Tox System (version 4.2.2; Xybion Medical Systems Co., Cedar Knolls, NJ, USA) and SAS software version 9.1 (SAS Institute, Cary, NC, USA). Significant probability values are represented as
P<0.05 (*) and
P<0.01 (**).
Results
Clinical signs and mortality
There were no treatment-related mortalities in any of the animals treated with 1,4-DCB during the study period (
Table 1). However, treatment-related clinical signs, including salivation, decreased locomotor activity, loss of fur, abnormal fur, and closed eyes were observed at a high incidence in the 1,000 mg/kg/day group. Post-dosing salivation was also observed in the 100 and 300 mg/kg/day groups at a high incidence.
Body weight changes
As shown in
Table 2, the body weight of male rats was significantly suppressed in the 1,000 mg/kg/day group on days 4, 8, 11, and 15 when compared with that in the control group.
Hematology
No significant differences between the treatment groups and controls were observed for any of the hematological parameter examined (
Table 3).
Serum biochemistry
As presented in
Table 4, a significant increase in AST level was observed in the 100 mg/kg/day group in comparison with that in the control group (
Table 4). The 1,000 mg/kg/day group exhibited a significant increase in ALT, ALP, T-CHO, T-BIL, ALB, PL, BUN, and GGT levels when compared with those in the control group.
Gross findings
At the scheduled necropsy, no treatment-related gross findings were observed in any of the treated animals at necropsy (data not shown).
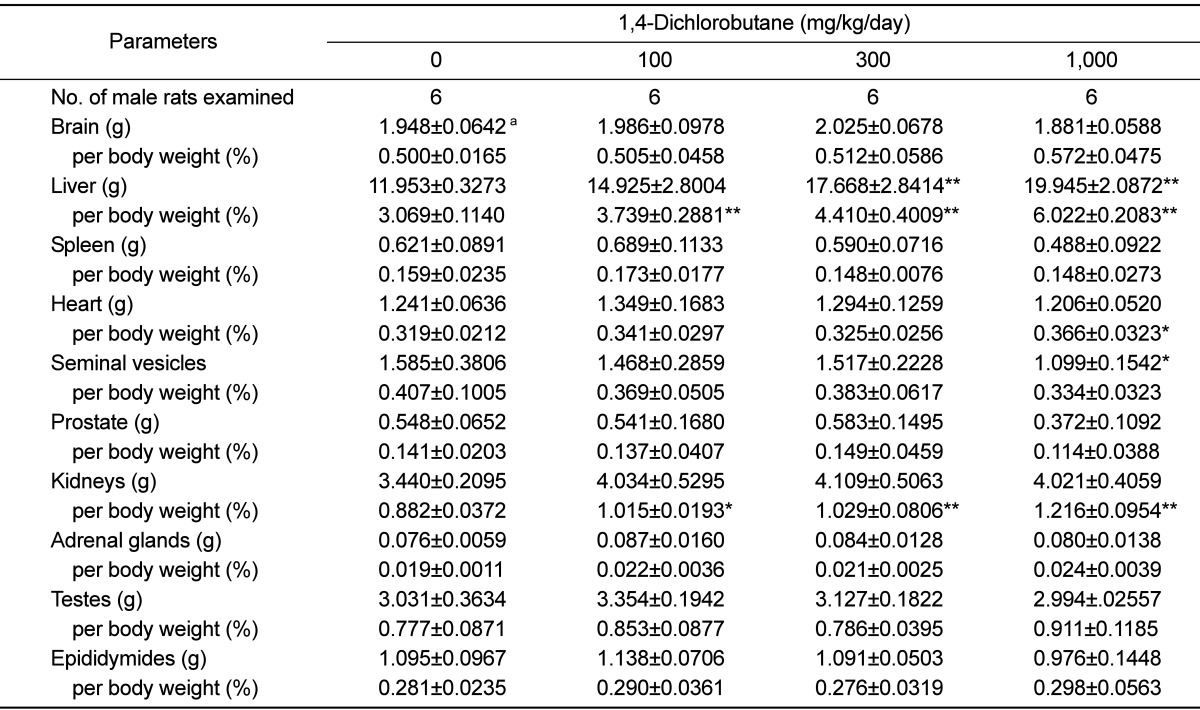
Organ weights
The absolute and relative organ weights of the rats are shown in
Table 5. A significant increase in the relative weights of liver and kidneys were observed in the 100 mg/kg/day group compared with those in the control group. A significant increase in the absolute and relative weights of the liver and relative weight of the kidneys was observed in the 300 mg/kg/day group compared with those in the control group. In the 1,000 mg/kg/day group, the absolute and relative weights of the liver and the relative weights of the heart and kidneys were significantly increased, whereas the absolute weight of seminal vesicles was significantly increased when compared with those in the control group.
Discussion
The present study investigated the potential subacute toxicity of 1,4-DCB by a 4-week repeated oral administration to Sprague-Dawley rats at dose levels of 0, 100, 300, and 1,000 mg/kg/day. The results of this study showed that daily oral administration of 1,4-DCB for 4 weeks at 1,000 mg/kg/day caused some changes in clinical signs, body weight, serum biochemical parameters, and organ weights in rats.
Treatment-related clinical signs were observed in the 1,000 mg/kg/day group, as evidenced by dose-dependent increases in the incidence and severity of salivation, decreased locomotor activity, loss of fur, abnormal fur, and closed eyes. An increase in the incidence of post-dosing salivation was observed in the low and middle-dose groups, indicating that it was also caused by the administration of 1,4-DCB as it was not observed before treatment and was observed only immediately after treatment. This finding may be attributed to oral mucosa and gastrointestinal irritation effects of 1,4-DCB because this chemical acts as a skin, eye, and respiratory system irritant [
1]. It is well known that salivation is often observed in gavage studies and may be a reaction to the taste or irritation of the test article rather than an indication of toxicity [
6,
7]. Therefore, it was considered that the transient salivation observed in this study was of doubtful toxicological significance, since no related changes were observed in the low and middle-dose groups.
The significant suppression in body weight gain observed in the 1,000 mg/kg/day group was considered to be related to the 1,4-DCB treatment, as an increased incidence and severity of clinical signs was observed in the group during the study period. This was a clear indication of the general toxicity induced by 1,4-DCB treatment. Although the difference was not statistically significant in comparison with the controls during the last 10 days, the suppression of body weight gain in the high dose group was consistently observed until the end of the experiment period.
1,4-DCB, at doses up to 1,000 mg/kg, did not cause any hematological abnormalities in the animals tested. It has been also reported that 1,4-DCB at a dose of 2,000 mg/kg does not induce micronuclei in bone marrow cells of ICR mice [
1]. In contrast, a 4-week repeated oral administration of 1,4-DCB at 1,000 mg/kg/day resulted in treatment-related abnormalities in serum biochemical parameters. Serum aminotransferase activity is one of the most frequently relied upon laboratory indicators of hepatotoxic effects [
8,
9]. Injury to hepatocytes alters their transport function and membrane permeability, leading to leakage of enzymes from the cells [
10]. Therefore, the marked release of ALT into circulation indicates severe damage to hepatic tissue membranes. Serum ALP and GGT activity is a reliable maker of hepatobiliary injury and cholestasis [
11-
13], and elevated levels of these enzymes may affect T-CHO and T-BIL levels. The serum biochemical changes observed in the 1,000 mg/kg/day group, including a significant increase in serum ALT, ALP, T-CHO, T-BIL, PL, BUN, and GGT values were considered to be treatment-related effects because these findings exhibited a clear-cut dose-response relationship, and the findings were consistent with the increased kidney and liver weights in the group. In contrast, the significant increase in AST observed in the 100 mg/kg/day group was not considered to be treatment related. This is because the changes did not exhibit a dose-response relationship and were within the limits of normal biological variations [
14-
16].
It is well known that the changes in organ weights are a sensitive indicator of the detection of potentially toxic chemicals [
17]. The dose-dependent increases in relative organ weights with increasing dosage indicate that the relative weights of the organs were closely related to the 1,4-DCB treatment. Of the organ weight changes observed in this study, the increased weights of the liver and kidneys in the 1,000 mg/kg/day group were of toxicological significance because these changes were remarkable and were accompanied by serum biochemical alterations, suggesting that the liver and kidneys are major targets of 1,4-DCB in rats. However, the increased relative heart weight and decreased absolute weight of the seminal vesicles observed in the 1,000 mg/kg/day group were not considered toxicologically significant because they were considered a result of a reduction in body weight and did not show a dose-response relationship. The significant increase in liver and kidney weights in the 100 and 300 mg/kg/day groups were also of doubtful toxicological significance, as these changes were unaccompanied by correlated serum biochemical changes.
In conclusion, the 4-week repeated oral administration of 1,4-DCB to rats resulted in a decrease in body weight gain and increases in ALT, ALP, T-CHO, T-BIL, PL, BUN, GGT levels, kidney and liver weights at 1,000 mg/kg/day. The target organs of 1,4-DCB were determined to be liver and kidney in rats. The no-observed-adverse-effect level was considered to be 300 mg/kg/day in rats. Information on the potential subacute toxicity of 1,4-DCB after repeated oral exposure is still not available. Therefore, the present results are expected to provide information on the general toxic effects and target organ toxicity of 1,4-DCB via repeated oral exposure to aid in the risk assessment process.
Acknowledgments
This study was supported by a research fund from the Korea Occupational Safety and Health Agency. This work was also supported by a grant from the Animal Medical Institute of Chonnam National University.
References
1. Rim KT, Kim SJ, Kim JK, Chung YH, Park SY, Yang JS. In vivo micronucleus test of methylcyclopentane and 1,4-dichlorobutane. J Korea Soc Environ Anal. 2011; 14:89–93.
2. Bartlett MS. Properties of sufficiency and statistical test. Proc R Soc London Series A. 1937; 160:268–282.
3. Kruskal WH, Wallis WA. Use of ranks in one-criterion analysis of variance. J Am Stat Assoc. 1952; 47:583–621.
4. Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 1964; 20:482–491.

5. Fisher RA. Statistical Methods for Research Workers. 1970. 14th ed. London: Oliver and Boyd Limited;p. 354.
6. Kim JC, Shin DH, Ahn TH, Kang SS, Song SW, Han J, Kim CY, Ha CS, Chung MK. 26-Week oral repeated dose toxicity study of the new quinolone antibacterial DW-116 in Sprague-Dawley rats. Food Chem Toxicol. 2003; 41(5):637–645. PMID:
12659716.
7. Yu WJ, Chung MK, Chung YH, Kim HC, Kim SH, Lee IC, Kim JC. One-generation reproductive toxicity study of 2-methylbutane in Sprague-Dawley rats. Regul Toxicol Pharmacol. 2011; 60(1):136–143. PMID:
21419187.

8. Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol. 1998; 27(2):119–130. PMID:
9671567.

9. Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008; 245(3):194–205. PMID:
18291570.

10. Zimmerman HJ, Seeff LB. Coodley EL, editor. Enzymes in hepatic disease. Diagnostic enzymology. 1970. Philadelphia: Lea and Febiger;p. 1–38.
11. Sheehan M, Haythorn P. Predictive values of various liver function tests with respect to the diagnosis of liver disease. Clin Biochem. 1979; 12(6):262–263. PMID:
43785.

12. Leonard TB, Neptun DA, Popp JA. Serum gamma glutamyl transferase as a specific indicator of bile duct lesions in the rat liver. Am J Pathol. 1984; 116(2):262–269. PMID:
6147091.
13. Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol. 2007; 45(9):1551–1557. PMID:
17658209.

14. Kang BH, Son HY, Ha CS, Lee HS, Song SW. Reference values of hematology and serum chemistry in Ktc: Sprague–Dawley rats. Korean J Lab Anim Sci. 1995; 11:141–145.
15. Petterino C, Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol. 2006; 57(3):213–219. PMID:
16343876.

16. Wolford ST, Schroer RA, Gohs FX, Gallo PP, Brodeck M, Falk HB, Ruhren R. Reference range data base for serum chemistry and hematology values in laboratory animals. J Toxicol Environ Health. 1986; 18(2):161–188. PMID:
3712484.

17. Kim JC, Shin DH, Kim SH, Kim JK, Park SC, Son WC, Lee HS, Suh JE, Kim CY, Ha CS, Chung MK. Subacute toxicity evaluation of a new camptothecin anticancer agent CKD-602 administered by intravenous injection to rats. Regul Toxicol Pharmacol. 2004; 40(3):356–369. PMID:
15546689.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download