Abstract
Red Liriope platyphylla (RLP) has been manufactured from Liriope platyphylla (L. platyphylla, LP) roots using steaming process and investigated as a curative agent for treatment of diabetes, obesity and neurodegenerative disorders. To examine the precautionary effects of aqueous extract RLP (AEtRLP) on the preclinical stages of Alzheimer's Disease (AD), alterations of the key factors influencing AD were investigated in Tg2576 mice after AEtRLP7 treatment for 4 months. Aβ-42 peptides level was significantly decreased in the brain of AEtRLP7-treated Tg2576 mice compared to vehicle-treated Tg2576 mice, although significant differences on improving behavioral defects were not observed in the same group. The concentration of nerve growth factor (NGF) in serum was also higher in AEtRLP7-treated Tg2576 mice than vehicle-treated Tg2576 mice. However, the phosphorylation of TrkA and Erk among the downstream effectors of the high affinity NGF receptor was significantly lower in AEtRLP7-treated Tg2576 mice. A similar pattern was observed in the expression level of downstream effectors within low affinity NGF receptor. Overall, these results suggest that AEtRLP7 can contribute to preventing the production and deposition of Aβ-42 peptides during the early progression stage of AD in the brain of Tg2576 mice through increased NGF secretion.
RLP was first manufactured by steaming, which is often applied to medicinal herbs to enhance the concentration or efficacy of functional components and induce chemical transformations in therapeutic components [1,2]. Among products prepared under various steaming conditions, aqueous extract of RLP9 (AEtRLP9) manufactured with two repeated steps (3 h steaming followed by 24 h of air-drying) carried out nine times induced maximum insulin secretion in INS cells without any effects on cell viability [2]. Additionally, AEtRLP9 treatment applied for 14 days downregulated glucose concentration and upregulated insulin concentration in a type I diabetic model, which had the diabetic condition induced by streptozotocin treatment [2]. Furthermore, study about the effects of AEtRLP9 in type II diabetic model, OLETF rats, showed a significant increase of insulin secretion and adiponectin concentration, a decrease of fatty liver formation and recovery of β-oxidation genes in fat tissue [3]. Moreover, the intracellular calcium concentration was also higher in AEtRLP9 treated INS-1 cells than in an LP-treated group, although this elevation was abrogated in response to administration of 40 mM nifedipine in a dosedependent manner [4].
RLP is also tightly associated with NGF secretion ability and the NGF receptor signaling pathway. The maximum NGF secretion was detected in neuroblastoma cells treated with aqueous extract of RLP7 (AEtRLP7) manufactured using two repeated steps (3 h steaming and 24 h of air-drying) that were conducted seven times. In addition, AEtRLP7 treatment altered downstream effectors expression of NGF receptors and extracellular and intracellular calcium levels [5]. NSE/APPsw transgenic mice treated with AEtRLP7 for 3 weeks showed increased NGF concentration in serum, the suppression of downstream effectors expression in the NGF receptor signaling pathway, and reduced Aβ-42 peptides level [6]. Despite these primary results, it is still not clear whether AEtRLP7 treatment of Tg2576 mice at the preclinical stage prevents induction of the pathological phenotypes of AD.
Therefore, this study investigated the effects of AEtRLP7 on neurodegenerative disorder-related factors, including NGF secretion ability, the NGF receptor signaling pathway, and Aβ-42 production, as well as the potential to use AEtRLP7 as a preventive medicine for treatment of neuronal-related diseases.
AEtRLP7 was prepared as described in previous studies [2]. Briefly, fresh roots of LP were collected from plantations in the Miryang (Korea) and sufficiently dried in a hot-air drying machine (JSR, Seoul, Korea) at 60℃. The voucher specimens of LP (WPC-11-010) were deposited at the Functional Materials Bank of PNU-Wellbeing RIS Center in Pusan National University. To produce RLP7 at seven different steaming frequencies, 200 g of dry roots were steamed at 99℃ for 3 h and then air-dried at 70℃ for 24 h. These two steps were carried out a total of seven times, after which the final produced RLP7 was reduced to powder using an electric blender. Next, the AEtRLP7 was purified for 2 h at 100℃ using circulating extraction equipment (IKA Labortechnik, Staufen, Germany) after adding 200 mL of distilled water into RLP7 powder. A solution of the extracts was also concentrated to dry pellets in a rotary evaporator (EYELA, Tokyo, Japan) and stored at -80℃ until needed. The yield of AEtRLP7 was about 86.22%.
Meanwhile, the composition of AEtRLP had been analyzed in our previous study [3]. They consisted of carbohydrates (83.22%), moisture (8.24%), crud protein (8.24%), crud ash (2.66%) and crud fat (0.94%). Especially, HPLC chromatogram showed large amount of polyphenolic compounds which have the antioxidant activity contained in RLP when compared with the LP.
The animal protocol used in this study was reviewed and approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2011-00220). Adult Tg2576 mice and Non-Tg mice were purchased from Samtaco (Osan, Korea) and handled at the Pusan National University Laboratory Animal Resources Center according to the National Institutes of Health guidelines. All mice were provided with a standard irradiated chow diet (Purina Mills Inc., Seoungnam, Korea) ad libitum, and were maintained in a specific pathogen-free state under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23±2℃ and a relative humidity of 50±10%.
Three-month-old Tg2576 mice (n=9) were assigned to either vehicle-treated group or AEtRLP7-treated group (n=4-5). Mice in the AEtRLP7-treated group were fed 50 mg/kg/day AEtRLP7 via their drinking water, while mice in the vehicle-treated group received distilled water. Non-Tg littermates were used as a control group. Following AEtRLP7 treatment for 4 months, all animals were immediately sacrificed using CO2 gas, after which blood and tissue samples were collected and stored in Eppendorf tubes at -70℃ until assayed.
To identify Tg2576 mice, genomic DNA isolated from mouse tails was genotyped by polymerase chain reaction (PCR) analysis. For genomic DNA-PCR, 10 pmol each of APPSW-specific primers, sense: 5'-CTG ACC ACT CGA CCA GGT TCT GGG T-3' and antisense: 5'-GTG GAT AAC CCC TCC CCC AGC CTA GAC CA-3', were added into genomic DNA template mixtures, after which the reaction mixtures were subjected to 25 cycles of amplification. Amplification was conducted in a T100 thermal cycler (BioRad Laboratories Inc., Hercules, California, USA) under the following conditions: denaturation for 30 s at 94℃, annealing for 30 s at 62℃, and extension for 45 s at 72℃. The amplified PCR products were then loaded onto a 1.0% agarose gel, after which the bands were detected using the Kodak Electrophoresis Documentation and Analysis System 120 (Eastman Kodak, Rochester, NY, USA).
Passive avoidance test was conducted as the method described by Fujiwara [7]. The apparatus (490×250×300 mm3, DJ-369, Daejong Inc., Seoul, Korea) used for the passive avoidance test consisted of an illuminated and a dark compartment separated by an acryl plate with a small passage. During the learning stage, a mouse was placed in the illuminated compartment. While this compartment was lit, the mouse stepped through the opened a passage in to the dark compartment. The time spent in the illuminated compartment was defined as the latency period. At three seconds after entering the dark compartment, a foot shock (0.01 mA, 200 V) was delivered to the floor grid in the dark compartment. The mouse could only escape from the shock by stepping back into the safe illuminated compartment. Such acquisition trials during the learning stage were carried out once a day for 2 days, and the mouse was judged to have learned avoidance from the foot shock when the latency period reached 300 s. Retention trials were carried out once every other month for 4 months (3, 5 and 7 months) to evaluate the retention of avoidance memory. To accomplish this, latency was measured for up to 300 s without delivering a foot shock, and a mouse was considered to have retained avoidance memory when it stayed in the illuminated safe compartment for 300 s. All retention tests were conducted between 10:00 a.m. and 12:00 p.m.
The levels of NGF in sera collected from Non-Tg, vehicle- and AEtRLP7-treated Tg2576 mice were measured using a NGF ELISA kit (Chemicon International Inc., Temecula, CA, USA). Briefly, the sample and standards were incubated overnight on antibody-coated plates in a plate shaker at 100-150 rpm at 2-8℃. The wells were then washed four times with washing buffer, after which 100 µL of anti-mouse NGF monoclonal antibody was added to each of the wells. Plates were subsequently incubated in a shaker for 2 h at room temperature, after which 100 µL of peroxidase-conjugated donkey anti-mouse IgG polyclonal antibody was added to each well and samples were incubated at room temperature for 2 h. After washing, 100 µL of TMB/E substrate was added to each well and the plate was incubated at room temperature for 15 min. The reaction was then quenched by the addition of 100 µL of stop solution, after which the plates were analyzed by a SoftMax Pro5 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Proteins prepared from the brain tissues of Non-Tg, vehicle- and AEtRLP-treated Tg2576 mice were separated by electrophoresis on a 10-20% SDS-PAGE gel for 2 h, then transferred to nitrocellulose membranes for 2 h at 40 V. Next, each membrane was incubated separately with one of the following primary antibodies: anti-TrkA antibody (Cell Signaling Technology, Beverley, MA, USA), anti-p-TrkA antibody (Cell Signaling Technology), anti-Akt antibody (Cell Signaling Technology), anti-p-Akt antibody (Cell Signaling Technology), anti-ERK antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p-ERK antibody (Santa Cruz Biotechnology), anti-p75NTR antibody (Cell Signaling Technology), anti-RhoA antibody (Cell Signaling Technology), anti-Bax antibody (Abcam, Cambridge, UK), anti-Bcl-2 (Abcam), or anti-beta actin (Sigma-Aldrich, St. Louis, MO, USA). Each membrane was then washed with buffer (137 mM NaCl, 2.7 mM KCl, 10 mM NaHPO4, and 0.05% Tween-20) and incubated with a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG at room temperature for 2 h. Finally, the membrane blots were developed using an Enhanced Chemiluminescence (ECL) Reagent Plus kit (Amersham Life Science, Piscatway, NJ, USA).
Brain perfusion and immunohistochemical analyses were performed as previously described [8,9]. Briefly, mice were anaesthetized by intraperitoneal injection of Zoletile (150 mg/kg body weight) and transcardially perfused with 1× PBS followed by 4% formaldehyde to effectively remove blood and fix brain tissue. Following perfusion, each mouse brain was isolated from the skull and fixed overnight in formaldehyde, after which each brain was dehydrated and embedded in paraffin. A series of brain sections (10 µm) were then cut from paraffin-embedded tissue using a Leica microtome (Leica Microsystems, Bannockbrun, IL, USA). For immunohistochemical analysis, these sections were deparaffinized with xylene, rehydrated, and then pretreated for 30 min at room temperature with PBS blocking buffer containing 10% goat serum. The sections were subsequently incubated with anti-Aβ-42 antibody (Invitrogen, Carlsbad, CA, USA) at a dilution of 1:100 in PBS blocking buffer. The antigen-antibody complexes were visualized using biotinylated secondary antibody (goat anti-rabbit)-conjugated HRP streptavidin (Histostain-Plus Kit; Zymed, South San Francisco, CA, USA) at a dilution of 1:1,500 in PBS blocking buffer. Aβ-42 peptides were detected using stable 3,3'-diaminobenzidine (DAB; Invitrogen) and observed using a BX50F-3 optical microscope (Olympus, Tokyo, Japan).
The levels of soluble Aβ-42 in brains of Non-Tg, vehicle- and AEtRLP7-treated Tg2576 mice were measured using a Human Aβ-42 ELISA kit (Invitrogen) according to the manufacturer's protocols. Briefly, the frontal lobe from the brain of each mouse was homogenized in ten volumes of guanidine-tris buffer (5.0 M guanidine HCl/50 mM Tris-HCl, pH 8.0), after which the homogenates were mixed for 3 h at room temperature and stored at 20℃ until analysis [10]. An Aβ-42 ELISA kit was used to measure the level of Aβ-42 in the brain homogenates according to the manufacturer's instructions. Briefly, the sample or standards and human Aβ-42 detection antibody solution were incubated on antibody-coated plates. Wells were then washed three times, after which HRP conjugate was added to each of the wells for 30 min. The reaction was terminated by the addition of 50 µL of stop solution, after which the plates were analyzed by evaluating the absorbance at 450 nm using a Molecular Devices Vmax Plate Reader (Sunnyvale, CA, USA) a SoftMax Pro5 spectrophotometer.
Tests of significance between the various types of Non-Tg mice and Tg2576 mice were carried out using One-Way ANOVA (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA). In addition, differences in the responses of the vehicle-treated group and AEtRLP7-treated group within Tg2576 mice were evaluated using a post-hoc test (SPSS for Windows, Release 10.10, Standard Version). All values are reported as the mean±standard deviation (SD), and a P value of<0.05 was considered significant.
For this test, the genotypes of Tg2576 mice were first identified by DNA-PCR analysis using genomic DNA isolated from the tails of 3-week-old mice. After electrophoresis, the PCR products of the APPSW construct were detected on 1.0% agarose gels as 466-bp bands (Figure 1A). The results suggested that all Tg2576 mice used in this study contained the APPsw gene in their genome.
Passive avoidance tests were carried out on mice at 3, 5 and 7 months of age to detect behavioral defects. In the first acquisition trial of the learning stage, all mice entered the dark compartment immediately after being placed in the illuminated compartment. Repeating the acquisition trial increased the latency times in all groups. All mice in each group acquired avoidance memory, staying in the illuminated compartment for more than 300 s at 3 and 5 months. No statistically significant differences were observed in the mean latency times among groups during the acquisition trials (Figure 1Ba and b). At 7 months, the latency time of Tg2576 mice was slightly lower than that of Non-Tg mice, while this level was recovered to those of the Non-Tg mice group after treatment with AEtRLP7. However, no significance difference was detected between the vehicle- and AEtRLP7-treated group (Figure 1Bc). Overall, these results show the possibility that AEtRLP7 treatment may affect the learning ability of Tg2576 mice after seven months old.
To investigate whether accumulation of Aβ-42 peptides could be suppressed by AEtRLP7 treatment, the concentration of Aβ-42 peptides was measured in the brain cortex of Tg2576 mice after treatment for 4 months. Immunohistochemical analysis showed that the number of Aβ-42 stained cells was significantly lower in the AEtRLP7-treated Tg2576 mice than in vehicle-treated Tg2576 mice (Figure 1C). Similar results were detected upon Aβ-42 ELISA. A significant decrease of soluble Aβ-42 concentration was observed in AEtRLP7-treated Tg2576 mice (Figure 1D). These results suggest that AEtRLP7 may contribute to a decrease in Aβ-42 concentration in the brain of Tg2576 mice.
To measure the effects of AEtRLP7 on the regulation of NGF secretion in Tg2576 mice, the concentrations of NGF were measured in the blood serum after AEtRLP7 treatment for 4 months. The level of NGF was lower in Tg2576 mice than Non-Tg mice. However, Tg2576 mice treated with AEtRLP7 showed 300% higher NGF concentrations than Tg2576 mice treated with vehicle alone (Figure 2A). These findings suggest that AEtRLP7 treatment for long periods of time could increase NGF secretion in the serum of Tg2576 mice.
Secreted NGF transduced the signal into the cytosol by binding two types of NGF receptors located on the cell membrane. Therefore, we investigated whether the increase in NGF concentration induced by AEtRLP7 treatment could affect the two NGF receptor signaling pathways in the brain of Tg2576 mice. In cases of analysis of the high affinity receptor, the level of TrkA phosphorylation was slightly higher in vehicle-treated Tg2576 mice than Non-Tg mice. However, this level was rapidly decreased by AEtRLP7 treatment for 4 months. The phosphorylation pattern of Erk, which is one of the downstream members activated by TrkA phosphorylation, was very similar to that of TrkA, although the rate of change varied in each group. However, the phosphorylation of the other TrkA downstream member, Akt, showed an opposite tendency from that of TrkA phosphorylation. In vehicle-treated Tg2576 mice, the level of Akt phosphorylation was lower than that of Non-Tg mice. However, after AEtRLP7 treatment for 4 months this level was slightly higher in the brain tissue of Tg2576 mice (Figure 3).
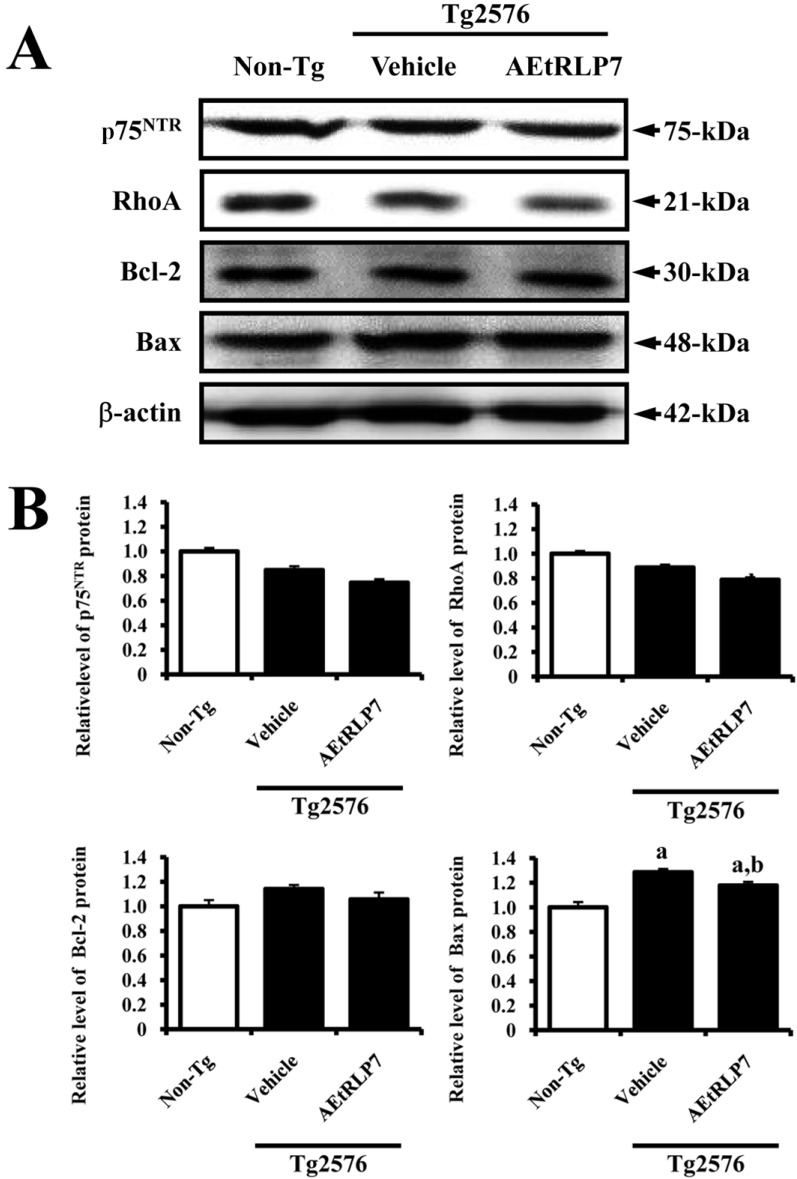
Conversely, in the case of low affinity receptor, the level of p75NTR and RhoA expression was lower in vehicle-treated Tg2576 mice than Non-Tg mice. After AEtRLP7 treatment, this level was lower in the brain tissue of AEtRLP-treated Tg2576 mice than vehicletreated Tg2576 mice. Also, the expression patterns of Bcl-2 and Bax, which are downstream members of p75NTR, were observed in the same mice. The level of Bcl-2 and Bax expression was decreased in the AEtRLP7-treated Tg2576 mice, but increased in the vehicle-treated Tg2576 mice (Figure 4). Therefore, the above results showed that the increased NGF induced by AEtRLP7 treatment could induce significant changes in the NGF receptor TrkA and p75NTR signaling pathways.
Tg2576 mice used in this study were produced by microinjecting the human APP695 gene containing the Swedish type mutation (K670N, M671L) under control of a hamster prion protein into B6SJLF2 fertilized eggs. These mice show AD patient-like phenotypes, including behavioral defects, a 14-fold increase in Aβ-42 peptide levels, and formation of numerous amyloid plaques at 9 to 10 months of age [11]. Therefore, these mice were considered the best model to investigate whether AEtRLP7 administration could prevent development of pathological phenotypes of neurodegenerative disorders. However, we used a model animal for AD at the preclinical stage (3 months of age) to investigate the precautionary effects of AEtRLP7. At this stage, Tg2576 mice did not show any specific symptoms related to AD, although the concentration of Aβ-42 peptides was significantly upregulated.
Tg2576 mice were also used to investigate the effects of dietary propentofylline (PPF) or acetyl-L-carnitine (ALCAR) treatment for 4 weeks on APP products and mRNA for NGF and NGF p75NTR receptor. PPF treatment induced an increase in the NGF mRNA level of 20%, while it decreased Aβ-40/42 peptides by 45/48%, with no effect on p75 NTR mRNA. Similarly, ALCAR increased p75 mRNA level by 16% and decreased Aβ-40/42 peptide by 45/48% [12]. Our findings regarding the NGF level are in agreement with those of previous studies, despite our detection method being different. However, in our study, the level of p75NTR protein expression decreased significantly in AEtRLP7 treated Tg2576 mice, which is very different from the results of previous studies in which the p75 mRNA level was increased or maintained in PPF or ALCAR treated Tg2576 mice. We believe that this difference can be attributed to differences in treatment compounds.
Some studies have reported the effects of traditional medicine on neurodegenerative disorders using a Tg2576 mouse model for AD. A traditional Japanese medicine, 1.0% yokukansan (TJ-54), was found to ameliorate learning deficits and non-cognitive defects, including anxiety, as well as to increase locomotor activity in Tg2576 mice [13]. Additionally, diet containing aged garlic extract (AGE)-containing and S-ally-L-cysteine (SAC) significantly upregulated the concentrations of synaptosomal-associated protein of 25-kDa (SNAP25) and synaptophysin in Tg2576 mice, while AGE protected 80% neuronal cells from ROS-mediated damage [14]. Moreover, NSE/APPsw Tg mice have been used to investigate the therapeutic effect of AEtRLP7 on neurodegenerative disorders [6]. In the present study, the precautionary functions of AEtRLP7 in brain were verified using Tg2576 mice at the preclinical stage. Our results showed that AEtRLP may contribute to the prevention of Aβ-42 peptides production in Tg2576 mice through increased NGF secretion.
Meanwhile, several studies demonstrated that exogenous NGF could improve or prevent the cholinergic neuron degeneration in the brains of rat and primate [15-19]. Especially, the cholinergic atrophy and spatial memory impairment in behaviourally impaired aged rats were partially ameliorated by the continuous intracerebral infusion of NGF over a period of four weeks [15]. However, in case of human patients, only two separate clinical trials have been performed to the intra-cerebroventricular (IVC) way of NGF. The patients treated with mouse NGF showed the increase of cortical blood flow and brain nicotine uptake, and improvement of verbal episodic memory [19,20]. Therefore, our results constitute the additional evidence that NGF treatment can alleviate the cholinergic neuron degeneration in the AD.
LP, a source material of RLP, also showed therapeutic potential in human subjects suffering from neurodegenerative disorders. The steroidal saponin spicatoside A isolated from LP induces neuritic outgrowth similar to NGF and activates extracellular signal-regulated kinase 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3-kinase/Akt) in PC12 cells [22]. Aqueous extract of LP induces increased the enhancement of NGF secretion, PC12 cell differentiation, and NGF mRNA expression in B35 and C6 cells [23]. Furthermore, NGF level in serum was significantly increased in NSE/hAPPsw Tg mice after AEtRLP7 treatment for 3 weeks, while Aβ-42 peptides were decreased in the same group [6]. The results of our study are in agreement with those of the aforementioned report, but the rate of increase and decrease varied. However, the main difference was that the current study used Tg2576 mice as a model of AD, whereas the aforementioned study utilized NSE/hAPPsw Tg mice. Furthermore, our study investigated the precautionary effects of AEtRLP7 in the preclinical stage of AD, while the aforementioned study was designed to investigate the therapeutic effects of AEtRLP7 in the clinical stage of AD. Although our results showed a meaningful data on the treatment of AD, more studies are needed to understand whether AEtRLP7 could prevent the accumulation of Aβ-42 peptide or the elimination of amyloid plaque through the detection of neprilysin expression in the brain of Tg2576 mice.
Overall, our study investigated the effects of AEtRLP7 on neurodegenerative disorder-related factors, including NGF secretion ability, NGF receptor signaling pathway, and Aβ-42 production. The results of our study revealed that AEtRLP7 has the potential for use as a preventive medicine for treatment of neuronal-related diseases.
References
1. Kim K, Kim HY. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008; 120(2):190–195. PMID: 18773949.

2. Choi SI, Lee HR, Goo JS, Kim JE, Nam SH, Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, Jang IS, Son HJ, Hwang DY. Effects of steaming time and frequency for manufactured Red Liriope platyphylla on the insulin secretion ability and insulin receptor signaling pathway. Lab Anim Res. 2011; 27(2):117–126. PMID: 21826171.
3. Lee HR, Kim JE, Goo JS, Choi SI, Hwang IS, Lee YJ, Son HJ, Lee HS, Lee JS, Hwang DY. Red Liriope platyphylla contains a large amount of polyphenolic compounds which stimulate insulin secretion and suppress fatty liver formation through the regulation of fatty acid oxidation in OLETF rats. Int J Mol Med. 2012; 30(4):905–913. PMID: 22842959.
4. Lee HR, Kim JE, Lee YJ, Kwak MH, Im DS, Hwang DY. Red Liriope platyphylla stimulated the insulin secretion through the regulation of calcium concentration in rat insulinoma cells and animal models. Lab Anim Res. 2013; 29(2):84–95. PMID: 23825481.
5. Choi SI, Goo JS, Kim JE, Nam SH, Hwang IS, Lee HR, Lee YJ, Son HJ, Lee HS, Lee JS, Kim HJ, Hwang DY. Differential effects of the steaming time and frequency for manufactured red Liriope platyphylla on nerve growth factor secretion ability, nerve growth factor receptor signaling pathway and regulation of calcium concentration. Mol Med Rep. 2012; 6(5):1160–1170. PMID: 22895564.
6. Choi SI, Goo JS, Kim JE, Hwang IS, Lee HR, Lee YJ, Son HJ, Lee HS, Lee JS, Hwang DY. Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and γ-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease. Lab Anim Res. 2012; 28(3):155–163. PMID: 23091515.
7. Fujiwara H, Takayama S, Iwasaki K, Tabuchi M, Yamaguchi T, Sekiguchi K, Ikarashi Y, Kudo Y, Kase Y, Arai H, Yaegashi N. Yokukansan, a traditional Japanese medicine, ameliorates memory disturbance and abnormal social interaction with anti-aggregation effect of cerebral amyloid β proteins in amyloid precursor protein transgenic mice. Neuroscience. 2011; 180:305–313. PMID: 21303686.

8. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124. PMID: 20193666.

9. Torres KC, Dutra WO, Gollob KJ. Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol. 2004; 65(11):1328–1335. PMID: 15556683.
10. Wang YJ, Pollard A, Zhong JH, Dong XY, Wu XB, Zhou HD, Zhou XF. Intramuscular delivery of a single chain antibody gene reduces brain Abeta burden in a mouse model of Alzheimer's disease. Neurobiol Aging. 2009; 30(3):364–376. PMID: 17686552.
11. Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996; 274(5284):99–102. PMID: 8810256.
12. Chauhan NB, Siegel GJ. Effect of PPF and ALCAR on the induction of NGF- and p75-mRNA and on APP processing in Tg2576 brain. Neurochem Int. 2003; 43(3):225–233. PMID: 12689602.

13. Tabuchi M, Yamaguchi T, Iizuka S, Imamura S, Ikarashi Y, Kase Y. Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of Alzheimer's disease. J Ethnopharmacol. 2009; 122(1):157–162. PMID: 19146938.

14. Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011; 117(3):388–402. PMID: 21166677.

15. Fischer W, Wictorin K, Björklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987; 329(6134):65–68. PMID: 3627243.

16. Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986; 6(8):2155–2162. PMID: 3746405.

17. Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987; 235(4785):214–216. PMID: 3798108.

18. Tuszynski MH, U HS, Amaral DG, Gage FH. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci. 1990; 10(11):3604–3614. PMID: 2230949.
19. Tuszynski MH, Sang H, Yoshida K, Gage FH. Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the adult primate brain. Ann Neurol. 1991; 30(5):625–636. PMID: 1763889.

20. Olson L, Nordberg A, von Holst H, Backman L, Ebendal T, Alafuzoff I, Amberla K, Hartvig P, Herlitz A, Lilja A, Lundqvist H, Langstrom B, Meyerson B, Persson A, Viitanen M, Winblad B, Seiger A. Nerve growth factor affects 11C-nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (case report). J Neural Transm Park Dis Dement Sect. 1992; 4(1):79–95. PMID: 1540306.
21. Eriksdotter Jönhagen M, Nordberg A, Amberla K, Bäckman L, Ebendal T, Meyerson B, Olson L, Seiger , Shigeta M, Theodorsson E, Viitanen M, Winblad B, Wahlund LO. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 1998; 9(5):246–257. PMID: 9701676.

22. Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009; 620(1-3):9–15. PMID: 19695245.

23. Choi SI, Park JH, Her YK, Lee YK, Kim JE, Nam SH, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. Effects of water extract of Liriope platyphylla on the mRNA expression and protein secretion of nerve growth factors. Korean J Med Crop Sci. 2010; 18(5):291–297.
Figure 1
Effects of AEtRLP7 treatment on behavioral defects and Aβ-42 peptides production in Tg2576 mice. (A) To identify the inserted APPsw gene in Tg2576 mice, genomic DNA isolated from the tails of founder mice was analyzed by PCR. The resulting products (466-bp) are shown. (B) Alterations of behavioral defects in Tg2576 mice were detected by passive avoidance test at 3, 5 and 7 months. (C) Production of Aβ-42 peptide in Non-Tg, vehicle- and AEtRLP7-treated Tg2576 mice was observed in brain sections stained with anti-Aβ-42 antibody. (D) The concentration of Aβ-42 peptide in the brain tissue of Tg2576 mice was measured using an anti-Aβ-42 ELISA kit. Data are reported as the mean±SD of three experiments. a, P<0.05 relative to Non-Tg mice. b, P<0.05 relative to vehicle-treated Tg2576 mice.
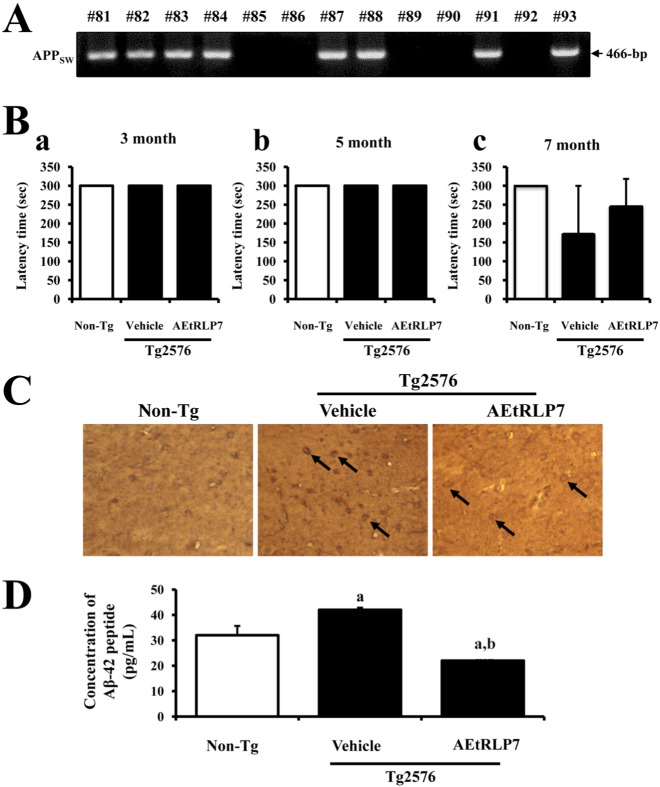
Figure 2
Alteration on NGF secretion of Tg2576 mice. The concentration of NGF in the serum of Tg2576 mice was measured using an anti-NGF ELISA kit. This kit has 10-15 pg/mL of sensitivity and can detect NGF at 10 pg/mL to 1,000 pg/mL. Data are reported as the mean±SD of three experiments. a, P<0.05 relative to Non-Tg mice. b, P<0.05 relative to vehicle-treated Tg2576 mice.
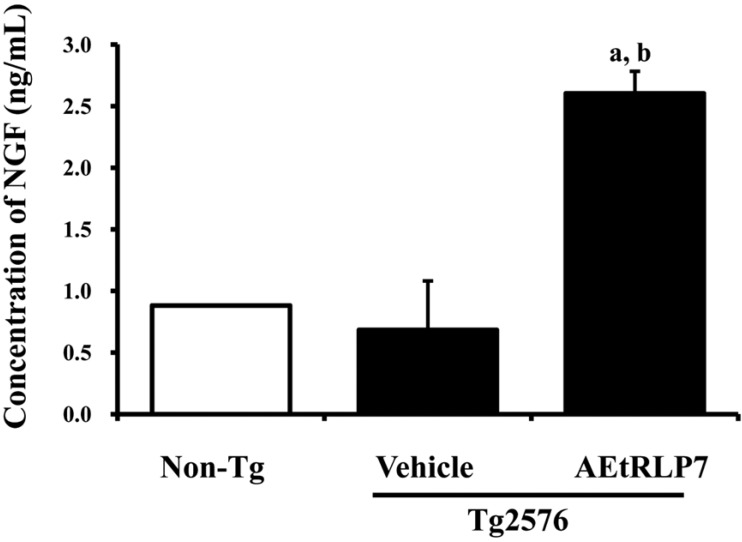
Figure 3
Effects of AEtRLP7 treatment on the NGF receptor TrkA signaling pathway of Tg2576 mice. Total tissue lysates were prepared from the brain of Tg2576 mice treated with vehicle or AEtRLP7 as described in the Materials and Methods section. Fifty micrograms of protein per sample were immunoblotted with antibodies for each protein. Three samples were assayed in triplicate by Western blotting. Data are reported as the mean±SD. a, P<0.05 relative to Non-Tg mice. b, P<0.05 relative to vehicle-treated Tg2576 mice.
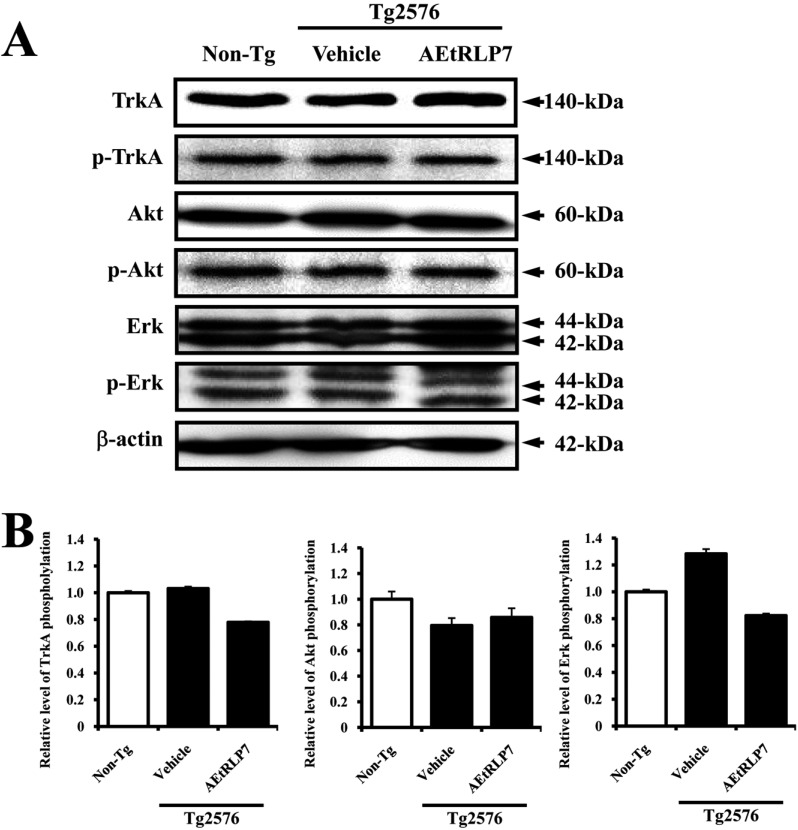
Figure 4
Effects of AEtRLP7 treatment on the NGF receptor p75NTR signaling pathway of Tg2576 mice. Total tissue lysates were prepared from the brain of Tg2576 mice treated with vehicle or AEtRLP7 as described in the Materials and Methods. Fifty micrograms of protein per sample was immunoblotted with antibodies for each protein. Three samples were assayed in triplicate by Western blot analysis. Data are reported as the mean±SD. a, P<0.05 relative to Non-Tg mice. b, P<0.05 relative to vehicle-treated Tg2576 mice.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download