Abstract
This study investigated the protective effects of diallyl disulfide (DADS) against cyclophosphamide (CP)-induced testicular toxicity in male rats. DADS was gavaged to rats once daily for 3 days at 100 mg/kg/day. One hour after the final DADS treatment, the rats were given a single intraperitoneal dose of 150 mg/kg CP. All rats were killed and necropsied on day 56 after CP treatment. Parameters of testicular toxicity included reproductive organ weight, testicular sperm head count, epididymal sperm motility and morphology, epididymal index, and histopathologic examinations. The CP treatment caused a decrease in body weight, testicular sperm head count, epididymal sperm motility, and epididymal index. The histopathological examination revealed various morphological alterations, characterized by degeneration of spermatogonia/spermatocytes, vacuolization, and decreased number of spermatids/spermatocytes in the testis, and cell debris and mild oligospermia in the ductus epididymis. In contrast, DADS pretreatment effectively attenuated the testicular toxicity caused by CP, including decreased sperm head count, epididymal sperm motility, and epididymal index and increased histopathological alterations in the testis and epididymis. These results indicate that DADS attenuates testicular toxicity induced by CP in rats.
Drugs used for cancer chemotherapy are often limited by severe acute toxic and undesirable side effects in multiple organ systems [1]. Thus, strategies to minimize the side effects of chemotherapeutic agents while preserving their chemotherapeutic efficacy are needed. Cyclophosphamide (CP) is a cytotoxic bifunctional alkylating agent that belongs to the nitrogen mustard class. It is extensively used as an antineoplastic agent for treating various cancers and as an immunosuppressive agent for organ transplantation [2-4]. However, despite its wide spectrum of clinical uses, CP causes several adverse effects including testicular toxicity in human and experimental animals [5-7].
Although the precise mechanism of CP-induced testicular toxicity is still not clearly understood, oxidative stress and the generation of toxic reactive oxygen species (ROS) have been implicated in the pathophysiology of CP toxicity [8-10]. It has been reported that oxidative DNA damage is caused by a hydroperoxide derivative of CP through generation of H2O2 [11]. Kothari et al. (2010) reported that ROS play a critical role in the pathogenesis of reproductive disorders, particularly in the pathological mechanism of male infertility [12]. Excess generation of ROS stimulates DNA fragmentation and a loss of sperm function associated with peroxidative damage to the mitochondria and plasma membrane [13]. However, antioxidant agents such as S-allylcysteine [9], melatonin [14], and DL-alpha-lipoic acid [15] have protective actions against CP-induced toxicity. Thus, the combination of drug delivery together with a potent antioxidant agent may be an appropriate approach to reduce CP side effects.
Garlic (Allium sativum L.) contains more than 20 organosulfur compounds and has been considered a dietary anticancer component. Among these compounds, diallyl disulfide (DADS) is a major component of the secondary metabolites derived from garlic and has been well documented as a potent compound to prevent cancer, urotoxicity, genotoxicity, nephrotoxicity, and hepatotoxicity [16-20]. Previous studies have shown that DADS is not only effective at modulating phase I enzymes and phase II enzymes [20-22] but also has potent antioxidant capacity [19,23]. Despite the favorable pharmacological properties of DADS, its protective capacity against testicular toxicity caused by CP has not been explored previously. Therefore, the present study was conducted to examine whether pretreatment with DADS prevents CP-induced testicular toxicity in rats.
Male Sprague-Dawley rats aged 12 weeks were obtained from a specific pathogen-free colony at Samtako Co. (Osan, Korea) and used after 1 week of quarantine and acclimation. Two rats per stainless wire mesh cage were housed in a room maintained at a temperature of 23±3℃ and a relative humidity of 50±10% with artificial lighting from 08:00 to 20:00 and with 13 to 18 air changes per hour. Rats were provided tap water that had been sterilized by ultraviolet irradiation and commercial rodent chow (Samyang Feed, Wonju, Korea) ad libitum. The Institutional Animal Care and Use Committee of Chonnam National University approved the protocols for the animals study, and the animals were cared for in accordance with the Guidelines for Animal Experiments of Chonnam National University.
CP was purchased from Sigma Aldrich Co. (St. Louis, MO, USA). DADS was purchased from Tokyo Kasei Chemical Co. (Tokyo, Japan). All other chemicals were of the highest grade commercially available. CP and DADS were dissolved in sterilized saline and corn oil, respectively, and were prepared immediately before treatment. The daily application volumes of CP (2 mL/kg body weight) and DADS (5 mL/kg body weight) were calculated based on the most recently recorded body weight of the individual animal. DADS was gavaged to rats once daily for 3 days at 100 mg/kg/day. One hour after the final DADS treatment, the rats were given a single intraperitoneal dose of CP (150 mg/kg). All animals were sacrificed 56 days after CP administration.
Twenty-four healthy male rats were randomly assigned to four experimental groups as follows: (1) vehicle control, (2) CP, (3) CP&DADS, and (4) DADS (n=6 per group). The CP dose was selected according to previous studies demonstrating significant damage in multi-organ systems of rats [24-26]. The effective DADS dose was based on earlier studies [17,27].
All rats were observed at least once daily for mortality and signs of reaction to the treatment. Body weights were measured weekly.
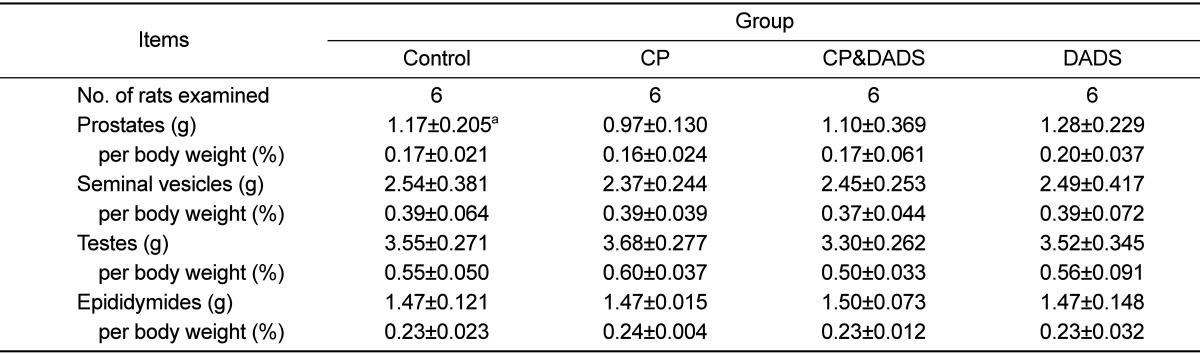
At the scheduled termination day (test day 56), all rats were killed by exposure to carbon dioxide and necropsied. The absolute weights of the prostates, seminal vesicles, testes, and epididymides were measured, and their weights relative to body weight were calculated.
A sperm analysis was conducted as described previously [28]. The left testis was homogenized and sonicated with 12 mL distilled water to calculate the sperm head count. The sperm suspension was loaded into a hemacytometer (Neubauer, Seligenstadt, Germany) and the number of homogenization-resistant sperm heads was counted under a light microscope (Leica, Wetzlar, Germany). For motility measurements, the sperm was obtained from the left cauda epididymis, placed in Hanks' balanced salt solution (pH 7.2) containing 5 mg/mL bovine serum albumin (Sigma) and maintained at 37℃. Motility was observed using a microscope with a stage warmer. Sperm were considered motile if they showed any movement at all. Smears of sperm suspensions were stained with 1% Eosin Y and allowed to air dry on a glass slide. Two hundred spermatozoa (intact sperm) per animal were evaluated for head and tail defects by light microscopy and were classified into the following categories: normal, small head, amorphous head, two heads/tails, straight hook, excessive hook, folded tail, short tail, and no tail.
The right testis and epididymis were taken and fixed in Bouin's solution. The tissues were routinely processed, embedded in paraffin, sectioned at 4-µm thickness, deparaffinized, and rehydrated using standard techniques. The sections were stained with hematoxylin-eosin stain for microscopic examination. All sections were examined with a light microscope by a pathologist blinded to the sample treatments.
The amount of sperm in the ductus epididymis was also semiquantitatively evaluated using a numerical scale where 3=normal, 2=slightly reduced (but >30% normal content), 1=markedly reduced (<30% of normal content), and 0=absent. This scale is referred to as the epididymal index.
Treatment-related clinical signs such as emaciation, decreased locomotor activity, anorexia, hematuria, polyuria, soft stool, alopecia, and diarrhea were observed in the CP group (data not shown). However, these signs diminished gradually during the experiment. Although treatment-related clinical signs were also found in the CP&DADS group, the rats in this group showed a tendency to decrease in the incidence and severity of the clinical signs. No clinical signs were observed in the control and DADS groups.
Figure 1 shows the effects of DADS on CP-induced changes in the mean body weight. Mean body weight on days 7 to 42 in the CP group was significantly lower than that in the control group. Mean body weight in the CP&DADS group also decreased significantly on days 7 to 21 when compared with that in the control group. Although no significant differences were observed between the groups, mean body weight on days 14 to termination in the CP&DADS group was slightly heavier than that in the CP group. Mean body weight in the DADS group was not difference when compared with that in the control group. As shown in Table 1, no significant differences were observed in the reproductive organ weights between the groups.
The results of the sperm examinations are summarized in Table 2. Testicular sperm head count and epididymal sperm motility in the CP group decreased significantly in comparison with those in the control group. In contrast, sperm motility in the CP&DADS group was significantly higher than that in the CP group. Although sperm head count in the CP&DADS group also decreased significantly in comparison with that in the control group, the DADS treatment resulted in a significant increase in sperm head count when compared with that in the CP group. Testicular sperm head count and epididymal sperm motility in the DADS group were no significantly difference when compared with those in the control group. No significant differences were observed in the morphological abnormalities of the sperm in any of the groups.
When the epididymis was evaluated for sperm content, epididymal index in the CP group decreased significantly in comparison with that in the control group. Although no significant differences were observed between the groups, the epididymal index in the CP&DADS group increased slightly compared to that in the CP group.
The results of the testicular histopathological examination are presented in Figure 2. The control (Figure 2A) and DADS groups (data not shown) presented testes with normal architecture. However, testicular tissue in the CP group showed various histopathological alterations, characterized by degeneration of spermatogonia/spermatocytes, vacuolization, and decreased number of spermatids/spermatocytes (Figure 2B and C). Although degeneration of spermatogonia and decreased number of spermatids/spermatocytes were also observed in the CP&DADS group (Figure 2D), the incidence and severity of histopathologic lesions were lower in comparison with those in the CP group.
The results of the epididymal histopathological examination are presented in Figure 3. The control and DADS groups presented epididymides with normal architecture (Figure 3A and D). However, the caudal epididymis in the CP group showed cell debris in ducts and mild oligospermia (Figure 3B). Although these findings were also observed in the CP&DADS group (Figure 3C), the incidence and severity of histopathologic lesions were lower in comparison with those in the CP group.
In the present study, we investigated the protective effects of DADS against CP-induced testicular toxicity in male rats 56 days after treatment. The design of the present study allowed for an estimation of the severity of stem cell destruction in rats. Fifty-six days is sufficient time for the surviving stem cells to progress through the differentiation pathway and produce a new generation of spermatozoa. This interval is also short enough to ensure that only a minimal regeneration of stem cell number occur [29]. The testicular sperm head count method can detect testicular lesions because CP causes damage mainly in cells at early phase of spermatogenesis [30,31]. Our results show that a single intraperitoneal dose of CP resulted in significant testicular toxicity, as evidenced by decreased sperm head count and sperm motility and testicular and epididymal histopathological alterations. However, the DADS treatment showed a protective effect against the testicular toxicity induced by CP in rats.
A number of reports have indicated that CP caused impairment of sperm and its fertilizing ability [6,7]. Adult patients treated with CP (1-2 mg/kg/day) for more than 4 months showed oligospermia or aspermia [5]. CP-exposed rats also have oligospermia and aspermia that manifest as biochemical and structural changes in the testis and epididymis [32,33]. CP produces two active metabolites such as phosphoramide mustard and acrolein. Lipid peroxidation (LPO) is one of the principal causes of CP-induced toxicity and is mediated by the production of acrolein, a metabolite for much of its toxicity [10,15,34]. Acrolein interferes with the tissue antioxidant defense system, produces highly ROS, and interacts with protein amino acids causing structural and functional changes in enzymes [35,36].
Oxidative stress plays a critical role in the pathogenesis of reproductive disorders and the etiology of defective sperm function [12]. Because the sperm plasma membrane contains a high content of polyunsaturated fatty acids and the cytoplasm is extremely limited in volume and localization, excess production of ROS is thought to damage spermatozoa through LPO, resulting in impaired metabolism, motility, acrosome reaction, and fusogenic capacity [37,38]. It has been reported that CP alters the sperm chromatin structure and the composition of sperm head basic proteins in rats [39]. In the present study, although there were no obvious differences in the incidence of abnormal sperm among the groups, the CP treatment significantly decreased epididymal sperm motility. In contrast, pretreatment with DADS improved sperm motility. These findings indicate that DADS effectively prevents spermatotoxicity induced by CP. A number of studies have demonstrated that the protective effect of S-allylcysteine (aged garlic extract) and garlic extract on CP toxicity is associated with preventing a rise in LPO and preserving antioxidant enzyme activities [9,40]. Additionally, garlic and organosulfur compounds from garlic have the cytoprotective and antioxidant activity against cadmium-induced testicular toxicity in rats [41,42]. Therefore, it is considered that the protective effects observed in this study may have resulted from the antioxidant activity of DADS.
The major histopathological findings observed in the CP group included degeneration of spermatocytes/spermatogonia, vacuolization, and decreased number of spermatids/spermatocytes in the testis and cell debris and mild oligospermia in the ductus epididymis. Histopathological alterations observed in the CP group may be considered a direct or indirect effect of CP, which later induces LPO as a chemical mechanism capable of disrupting the function of testis and epididymis. However, the incidence and severity of testicular and epididymal lesions in rats pretreated with DADS was considerably ameliorated when compared to that in the CP group. Therefore, DADS attenuated the CP-induced testis and epididymis damages.
The evaluation of testicular sperm head count seems to be a good indicator of spermatogenic damages and the testicular sperm head count corresponds to the number of elongate spermatids in the testis [43]. According to a previous study, the toxic effects of CP on spermatogenesis in the seminiferous tubules are considered one of the mechanisms of action of CP to produce dead sperm [32]. It has been also reported that the decrease in sperm head count induced by CP is due to the generation of ROS and the elimination of spermatogenic cells at different stages of development [15,31,44,45]. In the present study, a decrease in the testicular sperm head count was considered to result from the loss of early cell types caused by CP. Taken together, CP induced-testicular toxicity may be involved in the damage of differentiating spermatogenic cells during spermatogenesis. However, DADS pretreatment improved testicular toxicity, indicating that DADS effectively attenuates differentiating spermatogenic cells damaged by CP. The improved testicular toxicity obtained in rats treated with DADS was well correlated with the histopathological results and epididymal index. Kasuga et al. (2001) reported that garlic preparations are effective in recovery of spermatogenesis [46]. Recently, diallyl tetrasulfide from garlic has a protective effect against cadmium-induced testicular injury by reducing toxicant-mediated oxidative stress [47].
If ROS are mainly responsible for adverse effects of CP, antioxidants may actually reduce the severity of these adverse effects. It was previously reported that DADS pretreatment attenuates CP-induced urotoxicity and oxidative stress in mice [18]. Many investigators have observed that glutathione-related antioxidant defense systems are stabilized by DADS in the presence of oxidative stress [19,23,48]. Moreover, DADS exerts antioxidant protection by enhancing 3-hydroxy-3-methylglutaryl-CoA reductase, glutathione S-transferase, glutathione peroxides, and catalase [48-50]. The results of these studies and ours suggest that the antioxidant effects of DADS may be related to its ability to scavenge free radicals and ROS formed during CP intoxication.
In conclusion, DADS had protective effects against CP-induced testicular toxicity in rats. These findings suggest that DADS, which is a naturally occurring antioxidant from commonly consuming plants of allium spices, may be a useful protective agent against various testicular toxicities induced by oxidative stress.
Acknowledgments
This study was financially supported by Chonnam National University, 2012. The animal experiment in this study was supported by the Animal Medical Institute of Chonnam National University.
References
1. Sahin K, Sahin N, Kucuk O. Lycopene and chemotherapy toxicity. Nutr Cancer. 2010; 62(7):988–995. PMID: 20924974.

2. Baumann F, Preiss R. Cyclophosphamide and related anticancer drugs. J Chromatogr B Biomed Sci Appl. 2001; 764(1-2):173–192. PMID: 11817027.

3. Shanafelt TD, Lin T, Geyer SM, Zent CS, Leung N, Kabat B, Bowen D, Grever MR, Byrd JC, Kay NE. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer. 2007; 109(11):2291–2298. PMID: 17514743.

4. Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007; 7(9):2064–2074. PMID: 17614978.

5. Qureshi MS, Pennington JH, Goldsmith HJ, Cox PE. Cyclophosphamide therapy and sterility. Lancet. 1972; 2(7790):1290–1291. PMID: 4117814.

6. Elangovan N, Chiou TJ, Tzeng WF, Chu ST. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology. 2006; 222(1-2):60–70. PMID: 16517039.

7. Rezvanfar M, Sadrkhanlou R, Ahmadi A, Shojaei-Sadee H, Rezvanfar M, Mohammadirad A, Salehnia A, Abdollahi M. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum Exp Toxicol. 2008; 27(12):901–910. PMID: 19273545.

8. Manda K, Bhatia AL. Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol. 2003; 19(6):367–372. PMID: 15015761.

9. Bhatia K, Ahmad F, Rashid H, Raisuddin S. Protective effect of S-allylcysteine against cyclophosphamide-induced bladder hemorrhagic cystitis in mice. Food Chem Toxicol. 2008; 46(11):3368–3374. PMID: 18786597.

10. Motawi TM, Sadik NA, Refaat A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: an experimental study on rat myocardium, testicles and urinary bladder. Food Chem Toxicol. 2010; 48(8-9):2326–2336. PMID: 20573578.

11. Murata M, Suzuki T, Midorikawa K, Oikawa S, Kawanishi S. Oxidative DNA damage induced by a hydroperoxide derivative of cyclophosphamide. Free Radic Biol Med. 2004; 37(6):793–802. PMID: 15304255.

12. Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010; 48(5):425–435. PMID: 20795359.
13. Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004; 216(1-2):31–39. PMID: 15109742.

14. Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril. 2009; 92(3):1124–1132. PMID: 18829000.

15. Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. Beneficial effects of DL-alpha-lipoic acid on cyclophosphamide-induced oxidative stress in mitochondrial fractions of rat testis. Chem Biol Interact. 2005; 152(1):59–66. PMID: 15766923.
16. Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, Tsubura A. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001; 22(6):891–897. PMID: 11375895.

17. Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Mechanisms of protection against aflatoxin B(1) genotoxicity in rats treated by organosulfur compounds from garlic. Carcinogenesis. 2002; 23(8):1335–1341. PMID: 12151352.

18. Manesh C, Kuttan G. Alleviation of cyclophosphamide-induced urotoxicity by naturally occurring sulphur compounds. J Exp Clin Cancer Res. 2002; 21(4):509–517. PMID: 12636097.
19. Pedraza-Chaverrí J, González-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernández-Pando R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol. 2003; 473(1):71–78. PMID: 12877940.

20. Fukao T, Hosono T, Misawa S, Seki T, Ariga T. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem Toxicol. 2004; 42(5):743–749. PMID: 15046820.

21. Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun. 1998; 244(3):917–920. PMID: 9535768.

22. Guyonnet D, Siess MH, Le Bon AM, Suschetet M. Modulation of phase II enzymes by organosulfur compounds from allium vegetables in rat tissues. Toxicol Appl Pharmacol. 1999; 154(1):50–58. PMID: 9882591.

23. Wu CC, Sheen LY, Chen HW, Tsai SJ, Lii CK. Effects of organosulfur compounds from garlic oil on the antioxidation system in rat liver and red blood cells. Food Chem Toxicol. 2001; 39(6):563–569. PMID: 11346486.

24. Matsui H, Mitsumori K, Yasuhara K, Onodera H, Shimo T, Takahashi M. Morphological evaluation of cyclophosphamide testicular toxicity in rats using quantitative morphometry of spermatogenic cycle stages. J Toxicol Sci. 1995; 20(4):407–414. PMID: 8531236.

25. Abraham P, Indirani K, Sugumar E. Effect of cyclophosphamide treatment on selected lysosomal enzymes in the kidney of rats. Exp Toxicol Pathol. 2007; 59(2):143–149. PMID: 17686619.

26. Ayhanci A, Yaman S, Sahinturk V, Uyar R, Bayramoglu G, Senturk H, Altuner Y, Appak S, Gunes S. Protective effect of seleno-L-methionine on cyclophosphamide-induced urinary bladder toxicity in rats. Biol Trace Elem Res. 2010; 134(1):98–108. PMID: 19629405.

27. Wu CC, Sheen LY, Chen HW, Kuo WW, Tsai SJ, Lii CK. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J Agric Food Chem. 2002; 50(2):378–383. PMID: 11782211.

28. Kim KH, Shin IS, Lim JH, Kim SH, Park NH, Moon C, Kim SH, Shin DH, Kim JC. Dose-response effects of epichlorohydrin on male reproductive function in rats. Toxicol Res. 2009; 25:203–207.

29. Johnson FE, Farr SA, Mawad M, Woo YC. Testicular cytotoxicity of intravenous methotrexate in rats. J Surg Oncol. 1994; 55(3):175–178. PMID: 8176928.

30. Tates AD. Validation studies with the micronucleus test for early spermatids of rats. A tool for detecting clastogenicity of chemicals in differentiating spermatogonia and spermatocytes. Mutagenesis. 1992; 7(6):411–419. PMID: 1474916.

31. Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997; 56(6):1490–1497. PMID: 9166702.
32. Meistrich ML, Parchuri N, Wilson G, Kurdoglu B, Kangasniemi M. Hormonal protection from cyclophosphamide-induced inactivation of rat stem spermatogonia. J Androl. 1995; 16(4):334–341. PMID: 8537251.
33. Kaur F, Sangha GK, Bilaspuri GS. Cyclophosphamide-induced structural and biochemical changes in testis and epididymidis of rats. Indian J Exp Biol. 1997; 35(7):771–775. PMID: 9418379.
34. Lear L, Nation RL, Stupans I. Effects of cyclophosphamide and adriamycin on rat hepatic microsomal glucuronidation and lipid peroxidation. Biochem Pharmacol. 1992; 44(4):747–753. PMID: 1510722.

35. Haenen GR, Vermeulen NP, Tai Tin Tsoi JN, Ragetli HM, Timmerman H, Blast A. Activation of the microsomal glutathione-S-transferase and reduction of the glutathione dependent protection against lipid peroxidation by acrolein. Biochem Pharmacol. 1988; 37(10):1933–1938. PMID: 3377801.

36. Mythili Y, Sudharsan PT, Selvakumar E, Varalakshmi P. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact. 2004; 151(1):13–19. PMID: 15607758.
37. Aitken RJ, Harkiss D, Buckingham D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil. 1993; 98(1):257–265. PMID: 8345470.

38. Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995; 42(3):334–346. PMID: 8579848.

39. Codrington AM, Hales BF, Robaire B. Exposure of male rats to cyclophosphamide alters the chromatin structure and basic proteome in spermatozoa. Hum Reprod. 2007; 22(5):1431–1442. PMID: 17303633.

40. Unnikrishnan MC, Soudamini KK, Kuttan R. Chemoprotection of garlic extract toward cyclophosphamide toxicity in mice. Nutr Cancer. 1990; 13(3):201–207. PMID: 2308875.

41. Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol. 2008; 46(12):3604–3611. PMID: 18824205.

42. Sadik NA. Effects of diallyl sulfide and zinc on testicular steroidogenesis in cadmium-treated male rats. J Biochem Mol Toxicol. 2008; 22(5):345–353. PMID: 18972399.

43. Meistrich ML. Evaluation of reproductive toxicity by testicular sperm head counts. J Am Coll Toxicol. 1989; 8:551–567.

44. Schimenti KJ, Hanneman WH, Schimenti JC. Evidence for cyclophosphamide-induced gene conversion and mutation in mouse germ cells. Toxicol Appl Pharmacol. 1997; 147(2):343–350. PMID: 9439729.

45. Aguilar-Mahecha A, Hales BF, Robaire B. Effects of acute and chronic cyclophosphamide treatment on meiotic progression and the induction of DNA double-strand breaks in rat spermatocytes. Biol Reprod. 2005; 72(6):1297–1304. PMID: 15673603.
46. Kasuga S, Uda N, Kyo E, Ushijima M, Morihara N, Itakura Y. Pharmacologic activities of aged garlic extract in comparison with other garlic preparations. J Nutr. 2001; 131(3s):1080S–1084S. PMID: 11238821.

47. Ponnusamy M, Pari L. Protective role of diallyl tetrasulfide on cadmium-induced testicular damage in adult rats: a biochemical and histological study. Toxicol Ind Health. 2011; 27(5):407–416. PMID: 21245201.

48. Sheen LY, Sheu SF, Tsai SJ, Meng RH, Lii CK. Effect of garlic active principle, diallyl disulfide, on cell viability, lipid peroxidation, glutathione concentration and its related enzyme activities in primary rat hepatocytes. Am J Chin Med. 1999; 27(1):95–105. PMID: 10354821.

49. Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001; 131(3s):1010S–1015S. PMID: 11238807.

50. Yin MC, Hwang SW, Chan KC. Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic. J Agric Food Chem. 2002; 50(21):6143–6147. PMID: 12358493.

Figure 1
Changes in mean body weights of male rats treated with effects of DADS on CP-induced changes in the mean body weights. Statistical analysis was performed using one-way ANOVA followed by the Tukey's multiple comparison test. Data are expressed as means±SD. *P<0.05 compared with the control group. **P<0.01 compared with the control group.
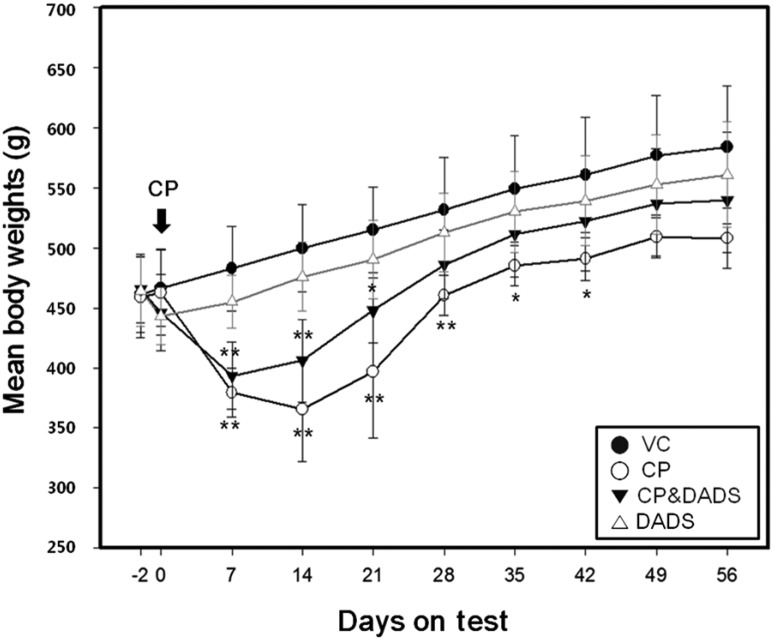
Figure 2
Representative photographs of testis sections treated with CP and/or DADS. Testis from vehicle (A) treated rats showing normal appearance. However, testis from a CP treated rat (B and C) showing degeneration of spermatocyte/spermatogonia (black arrow), vacuolization (white arrow), and decreased number of spermatids/spermatocytes (black arrow head). Testis from a CP&DADS treated rat (D) showing degeneration of spermatogonia. H&E stain. Bar=10 µm. 400.
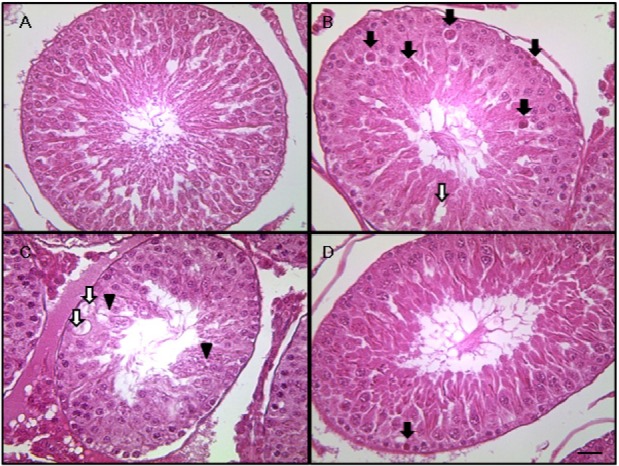
Figure 3
Representative photographs of epididymis sections treated with CP and/or DADS. Caudal epididymides from vehicle control (A) and DADS (D) treated rats showing normal luminal contents. However, caudal epididymis from a CP treated rat (B) showing cell debris in ducts and mild oligospermia. Caudal epididymis from a CP&DADS treated rat (C) showing mild cell debris in ducts. H&E stain. Bar=10 µm. 200.
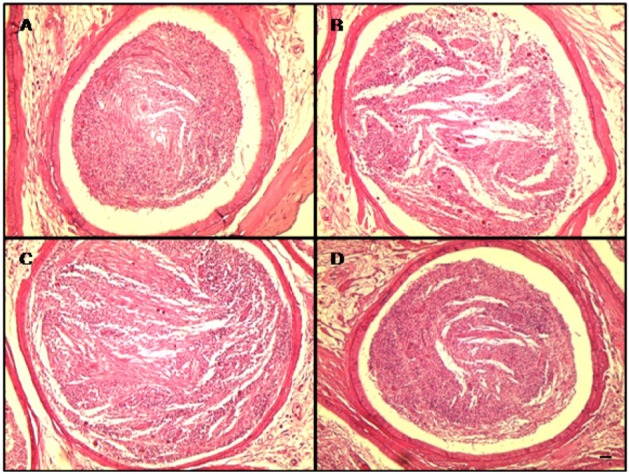
Table 2
Sperm analysis of male rats treated with CP and/or DADS
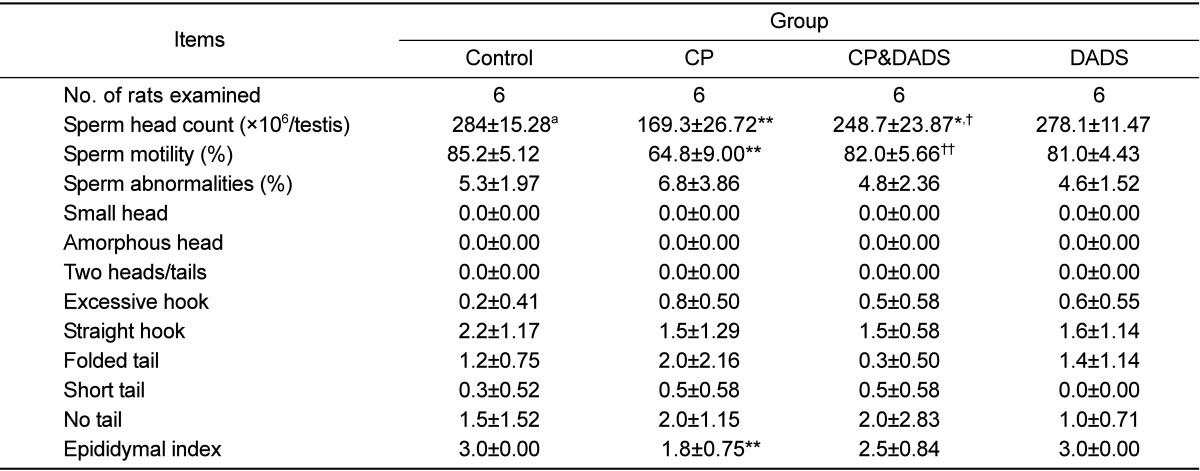
aValues are presented as means±SD.
*Significant difference at P<0.05 level compared with the control group.
**Significant difference at P<0.01 level compared with the control group.
†Significant difference at P<0.05 level compared with the CP group.
††Significant difference at P<0.01 level compared with the CP group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download