Abstract
This study was conducted to investigate the anti-fatigue effect of walnut extract (WE) on forced swimming capacity in mice. Twenty-eight male ICR mice were randomly divided into four groups, a vehicle control (VC) or one of three WE administered groups (300, 600 and 900 mg/kg/day). WE was orally administered to mice once a day for 4 weeks, during which time a forced swimming test was conducted once a week. The vehicle control group was given a corresponding volume of sterile distilled water. After 4 weeks, the forced swimming capacity and levels of blood lactate, glucose, glutamine, ammonia and triacylglycerol, and liver glycogen were measured. In the WE administration group (600 and 900 mg/kg) the maximum swimming time increased significantly when compared with the vehicle control group. WE (600 and 900 mg/kg) significantly decreased the levels of lactate andammonia and increased the blood glutamine levels and liver glycogen content after forced swimming relative to the vehicle control group. The results of this study demonstrated the anti-fatigue effects of WE in a dose-dependent manner. The effects of WE at 600 and 900 mg/kg were similar. Overall, these results suggest that walnut has anti-fatigue activity and could elevate exercise tolerance.
Go to : 
Fatigue is a complex phenomenon that can be defined as the inability to maintain expected muscle strength, leading to reduced performance during prolonged exercise. Fatigue is a time-dependent exercise-induced reduction in the maximal force generating capacity of a muscle [1]. Long-term accumulated fatigue can lead to death or severe health problems [2,3]. Since the currently available fatigue recovery therapies are very limited, potential alternatives from traditional medicineand herbal medicine are worth investigating [4]. Indeed, it was recently reported that various natural substances such as green tea extract [5,6] and red mold rice [7] are effective at preventing or reducing fatigue.
The forced swimming test is a widely-used experiment for rodents that has been employed to evaluate antifatigue treatments, and induces immobility as a reflection of fatigue when mice are placed in a deep water tank for an extended period [8].
Many epidemiological and clinical trials have demonstrated that frequent regular intake of walnuts is inversely correlated with myocardial infarction or mortality due to vascular ischemic disease, regardless of other risk factors such as age, obesity, hypertension, smoking and lack of exercise [9-11]. Comba et al. [12] reported that diets enriched with high levels of ω-9 fatty acids reduce tumor growth, metastasis and tumor leukocyte infiltration and that n-3, n-6 and n-9 polyunsaturated fatty acids alter growth and trigger apoptosis of breast cancer cells. Moreover, Ma et al. [13] reported that walnut-enriched diet improves endothelium-dependent vasodilatation in type 2 diabetic individuals, suggesting a potential reduction in overall cardiac risk. Walnut consumption has been recommended because walnuts have a high content of polyunsaturated fatty acids [9]. Indeed, walnuts are rich in α-linoleic, α-linolenic and γ-linolenic acids as well as other health-related compounds such as high-biological-value proteins, fibers, vitamins, tannins, folates and polyphenols (caffeic, p-coumaric, protocatechuic, vanillic, gallic, sinapic, p-hydroxybenzoic, chlorogenic and ellagic acids), flavonols (quercetin, isorhamnetin, kaempferol, morin) and/or their glycosides, flavanones (naringenin, eriodictyol) and/or their glycosides, flavan-3-ol monomers (catechin, epicatechin), dimers and trimmers [14]. However, the effects of walnuts on physical fatigue have not been thoroughly investigated. Therefore, this study was conducted to investigate the anti-fatigue effect of walnuts on forced swimming capacity in mice.
Male ICR mice (4 weeks old, weighing 18-22 g) were purchased from KOATEC (Osan, Korea). All mice were provided with free access to standard rodent chow (laboratory chow 5057, Purina Korea, Korea) and filtered tap water, and housed in an air-controlled room under standard conditions (temperature; 22±2℃, relative humidity; 50±10%, 12 hour light/dark cycle) throughout the study. After one week of acclimatization, the swimming times of all mice prior to walnut extract administration were measured.
The mice were randomly divided into four groups (n=7 in each group). Mice in the vehicle control (VC) group were administered distilled water (5 mL/kg body weight) by gavage every day for 4 weeks, while those in the other experimental groups were administered walnut extract (WE; 300, 600, or 900 mg/kg body weight) by gavage every day for 4 weeks. WE was dissolved in the same volume (5 mL/kg) of distilled water, and the doses of WE and 4 week treatment time used in this study were confirmed to be suitable and effective in ICR mice by previously conducted pilot tests. The amounts of food and water intake and body weight were measured once per week. In addition, blood samples were collected under ether anesthesia after measurement of the swimming time at the end of the experimental period. After blood sampling, the liver was removed, instantly frozen in liquid nitrogen, and stored at 80℃ until analysis. All animal experiments were performed in accordance with the Guidelines for Animal Care and Use Committee of Daegu Technopark, Biohealth Convergence Center (Approval No.: BCCIACUC-2011-03).
Walnuts (1 kg; cultivated in Young dong Province, Korea) were purchased at a domestic market. The whole body of the walnut was washed, segmented, soaked in 10 L of 70% ethanol for 24 h, filtered, lyophilized and powdered. Finally, 56 g of WE was prepared. The powdered WE was then stored -80℃. A voucher specimen was deposited in the veterinary toxicology laboratory of Kyungpook National University. The WE dosage (600 mg/kg/day) was based on the daily recommended intake as raw walnut (adult standard; 42 g/day) [15].
Forced swimming tests were conducted weekly using the method described by Ikarashi et al. [16] for 4 weeks. Tests were conducted in an acrylic pool (90×50×50 cm) filled with water (30±1℃) to a depth of 40 cm. Current in the pool was generated by a pump and maintained at a flow rate of 8 L/min. The mice were then made to swim until the end point of the swimming test, which was defined as the time at which the mice could not resist the current and failed to rise to the surface of the water to breathe within 7 s. In this study, the mice were subjected to a 10 min preliminary swim before 10 min of rest. After resting, the mice were made to swim until the end point of the swimming test, which was recorded as the swimming time.
Blood samples were taken by capillary tubes from the retro-orbital plexus of mice under light ether anesthesia 30 min after the forced swimming test. The serum was then separated by centrifugation (1000×g for 15 min) and stored at -80℃ until analyses. The serum glucose levels were quantified using a glucometer (Abbott Laboratories Medisense Products, Bedford, MA, USA). Serum lactate and triglyceride concentrations were determined using a commercial diagnostic kit according to the recommended procedures (Sigma Chemical Co., USA). Serum ammonialevels were quantified using a F-kit ammonia (Roche Diagnostics) and serum glutamine levels were quantified usinga glutamine assay kit(BioAssay Systems, Hayward, CA, USA).
After blood sampling, the mice were sacrificed, their livers were removed and the liver glycogen contents were then measured using a Glycogen Assay Kit (Sigma Chemical Co., USA).
All data are presented as the mean±SEM. The data were evaluated by one-way analysis of variance (ANOVA). Tukey's test for multiple comparisons was used to identify statistical differences between groups. A P<0.05 was considered to be statistically significant.
Go to : 
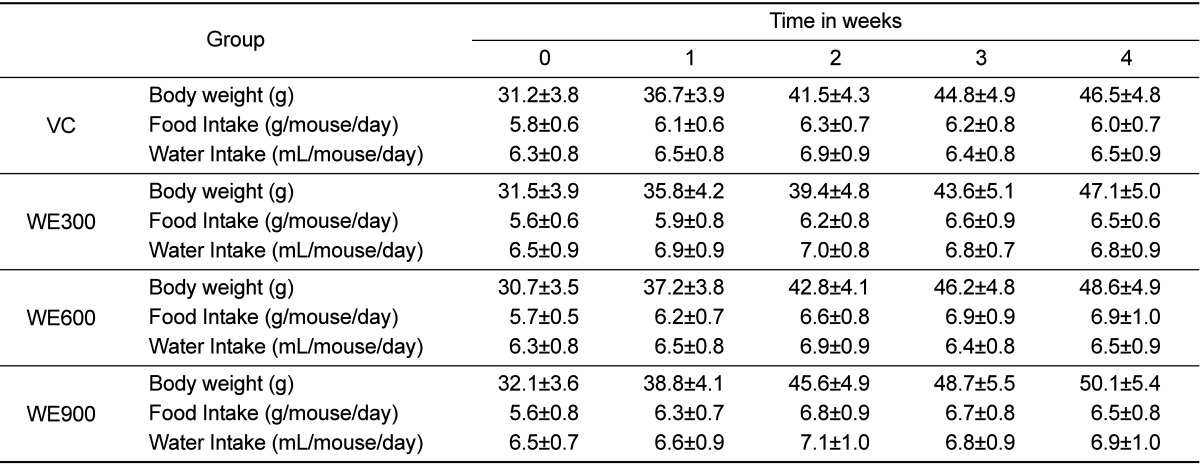
The body weight of mice at the initial point of the experiment was 31.2±3.8 g. There was no significant difference in body weight in WE treated mice when compared with the vehicle control animals after 4 weeks. The body weight, food intake, and water intake of all WE treated groups were similar to those of the vehicle control group (Table 1).
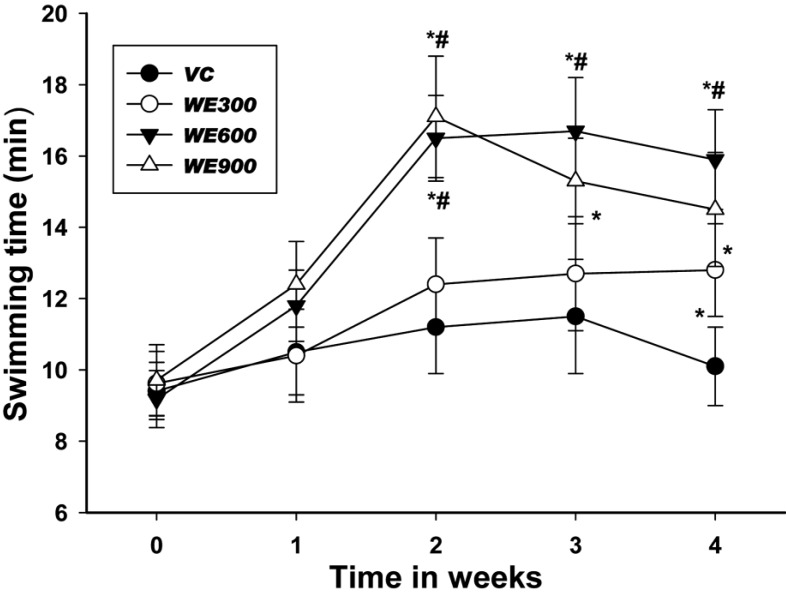
The effects of WE treatment on swimming time in mice for 4 weeks are shown in Figure 1. After 4 weeks, mice in the WE 300 mg/kg treatment group showed significantly increased swimming time when compared with the vehicle control group. Additionally, mice in the WE treatment (600 and 900 mg/kg) groups showed significantly increased the swimming time to exhaustion after 2 weeks when compared with those in the vehicle control and the 300 mg/kg treated group. No difference in swimming time was observed between the WE 600 and 900 mg/kg treated groups.
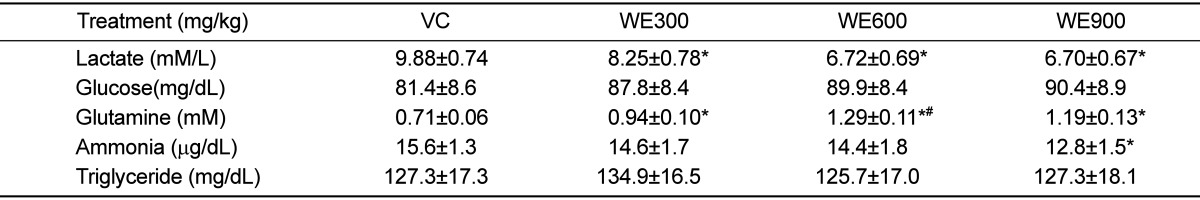
The serum components were analyzed after the last forced swimming test. The blood biochemical parameters are shown in Table 2. The blood lactate levels in all WE treated groups were lower than those in the vehicle control group, with significantly lower levels being observed in the 600 and 900 mg/kg WE treated groups. The blood glucose levels in all WE treated groups were slightly higher than those in the vehicle control group. However, no significant differences were observed in the blood glucose levels of the WE treated groups and the vehicle control group.
The blood glutamine levels in all WE treated groups were significantly higher than those in the vehicle control group, and these differences occurred in a dosedependent manner. The blood glutamine level in the 600 mg/kg WE treated group was highest, and was significantly higher than that of the 300 mg/kg WE treated group. The blood ammonia levels in all WE treated groups were lower than in the vehicle control group. The blood ammonia level in the 300 mg/kg WE treated group was similar to that in the vehicle control group, while the level in the 900 mg/kg WE treated group was significantly lower than that in the vehicle control group. The blood triglyceride concentrations in all WE treated groups were similar to that of the vehicle control group.
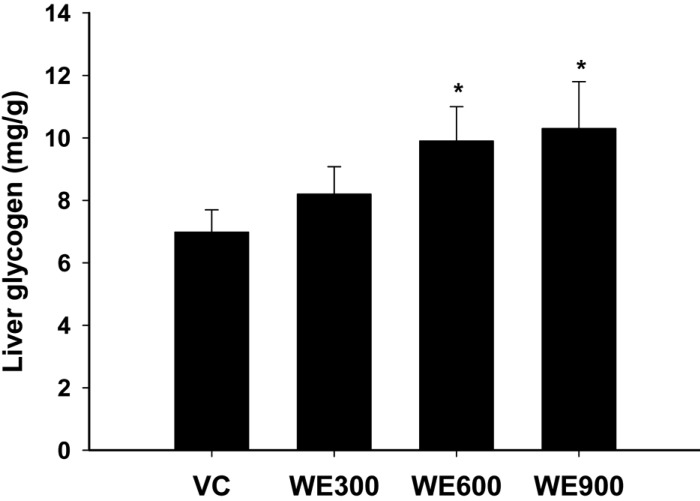
Figure 2 shows the changes in liver glycogen content in WE treated mice. The liver glycogen contents were enhanced by WE treatment in a dose-dependent manner. The liver glycogen contents in the 600 and 900 mg/kg WE treated group were significantly higher than those in the vehicle control group.
Go to : 
Walnuts are an excellent source of α-linolenic acid that have a high content of antioxidants including flavonoids, phenolic acid, melatonin, gamma tocopherol and selenium [14,17-18]. Walnuts have been used in traditional medicine for treatment of cardio-vascular insufficiency, atherosclerotic disorders, tumors, hemorrhoid, diarrhea, bacterial infection, fungal diseases, diabetes mellitus, hypertension and convulsion [19-21].
Little change was observed in the body weight, food and water intake of the WE treated groups after 4 weeks of administration, and no difference in the same parameters were observed among the WE treated groups (Table 1). In the present study, walnut intake did not cause weight gain, despite an increased energy intake, but the reasons for this are not clear. In experimental animals, polyunsaturated fatty acids caused less weight gain than saturated fats [22]. Although high in fat, walnut fats are mainly polyunsaturated and the effects of consuming walnuts on physical fatigue are unclear. In the present study, we confirmed that WE does not cause changes in the pattern of feeding.
The swimming times in all WE treated groups increased significantly relative to the vehicle control group after two weeks of treatment. However, there was no significant difference in the WE of the 300 mg/kg treated group except at four weeks (Figure 1). These results indicate that WE administration significantly enhanced the anti-fatigue capability of mice as indicated by the prolonged swimming time.
The levels of serum glucose, lactate, ammonia, and glutamine are known to serve as indicators of accumulated fatigue and stress caused by exercise [23]. Blood lactate is the glycolysis product of carbohydrate under anaerobic conditions, and glycolysis is the main energy source for short term intensive exercise. Because the accumulation of blood lactate causes fatigue during physical exercise, rapid removal of lactate is beneficial to relieving fatigue [24]. In the present study, the level of serum lactate was significantly lower following the administration of WE relative to the vehicle control group (Table 2). One of the main pathways to remove excess lactate is the conversion of lactate to glucose via gluconeogenesis, after which excess glucose is saved as a liver glycogen [25]. Therefore, the results of the study indicate that WE could decrease blood lactate level and alleviate physical fatigue.
The blood glucose levels in the WE treated groups were higher than in the vehicle control group. However, no significant difference was observed in blood glucose level between the WE treated group and the vehicle control group. These results suggest that WE did not significantly influence glucose metabolism factors, such as gluconeogenesis and glucose utilization in blood. We also found that blood glutamine levels and ammonia were significantly increased or decreased by the treatment of WE, respectively. It is assumed that WE may be attributable to the increase in glutamine synthesis in the liver. Vigorous exercise not only consumes glucose, but also glutamine, which is a primary amino acid for supplying energy in muscles [26]. Therefore, it is possible to enhance exercise capacity through WE administration via an increase in the concentration of glutamine in the blood.
Vigorous exercise also increases the production of ammonia, resulting in an increased level of ammonia in blood. This accumulated ammonia leads to decreased exercise capacity; however, it is possible to enhance exercise capacity by decreasing the concentration of ammonia in the blood [27]. Accordingly, these results suggest that WE has an anti-fatigue effect by increasing the activity of ammonia metabolism.
Liver glycogen increased remarkable by treatment with 600 and 900 mg/kg of WE when compared with the vehicle control group (Figure 2). The depletion of glycogen during high-intensity exercise severely limits energy supply and maximum power output [28]. The results of the present study showed that WE treatment enhanced the liver glycogen contents in a dosedependent manner. Because liver glycogen is the defense system of energy depletion, the enhancement of liver glycogen observed in this study indicated that WE could be utilized to serve as an energy source during vigorous exercise. Indeed, it is likely that WE contributes to the activation of energy metabolism in mice and thereby causes the prolongation of swimming time.
Finally, it should also be noted that WE intake did not increase blood triacylglycerol concentrations and that WE intake of 900 mg/kg per day for 4 weeks did not induce important changes in blood lipids in mice.
Based on these results, it is suggested that oral administration of WE of more than 600 mg/kg per day could significantly improve the endurance capability of mice to fatigue. However, further studies are necessary to clarify the detailed mechanism(s) involved in the antifatigue effects of WE.
Go to : 
Acknowledgment
This research was supported by Kyungpook National University Research Fund, 2011.
Go to : 
References
1. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001; 81(4):1725–1789. PMID: 11581501.

3. Ream E, Richardson A. Fatigue: a concept analysis. Int J Nurs Stud. 1996; 33(5):519–529. PMID: 8886902.

4. Tharakan B, Dhanasekaran M, Manyam BV. Antioxidant and DNA protecting properties of anti-fatigue herb Trichopus zeylanicus. Phytother Res. 2005; 19(8):669–673. PMID: 16177968.
5. Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I, Hase T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am J Physiol Regul Integr Comp Physiol. 2006; 290(6):R1550–R1556. PMID: 16410398.

6. Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol. 2006; 70(2):247–253. PMID: 15983804.

7. Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr, Jones RM, Portoghese PS, Carlezon WA Jr. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003; 305(1):323–330. PMID: 12649385.
8. Deyama T, Nishibe S, Nakazawa Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin. 2001; 22(12):1057–1070. PMID: 11749801.
9. Iwamoto M, Imaizumi K, Sato M, Hirooka Y, Sakai K, Takeshita A, Kono M. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr. 2002; 56(7):629–637. PMID: 12080402.

10. Feldman EB. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr. 2002; 132(5):1062S–1101S. PMID: 11983840.

11. Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004; 109(13):1609–1614. PMID: 15037535.
12. Comba A, Maestri DM, Berra MA, Garcia CP, Das UN, Eynard AR, Pasqualini ME. Effect of ω-3 and ω-9 fatty acid rich oils on lipoxygenases and cyclooxygenases enzymes and on the growth of a mammary adenocarcinoma model. Lipids Health Dis. 2010; 9:112. PMID: 20932327.

13. Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010; 33(2):227–232. PMID: 19880586.
14. Li L, Tsao R, Yang R, Liu C, Zhu H, Young JC. Polyphenolic profiles and antioxidant activities of heartnut (Juglans ailanthifolia var. cordiformis) and Persian walnut (Juglans regia L.). J Agric Food Chem. 2006; 54(21):8033–8040. PMID: 17032006.
15. Yoon MS. Effects of Walnut Extracts on cytokines in Mice Spleen with DNCB-induced Dermatitis. J Korean Soc Cosmetol. 2009; 15(3):1033–1040.
16. Ikarashi N, Fukazawa Y, Toda T, Ishii M, Ochiai W, Usukura M, Sugiyama K. Effect of Conclevan on endurance capacity in mice. Biol Pharm Bull. 2012; 35(2):231–238. PMID: 22293354.

17. Fukuda T, Ito H, Yoshida T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry. 2003; 63(7):795–801. PMID: 12877921.
18. Reiter RJ, Manchester LC, Tan DX. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition. 2005; 21(9):920–924. PMID: 15979282.

19. Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009; 90(1):56–63. PMID: 19458020.

20. Rajaram S, Haddad EH, Mejia A, Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr. 2009; 89(5):1657S–1663S. PMID: 19339404.

21. Davis PA, Vasu VT, Gohil K, Kim H, Khan IH, Cross CE, Yokoyama W. A high-fat diet containing whole walnuts (Juglans regia) reduces tumour size and growth along with plasma insulinlike growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. Br J Nutr. 2012; 108(10):1764–1772. PMID: 22244053.
22. Hill JO, Peters JC, Lin D, Yakubu F, Greene H, Swift L. Lipid accumulation and body fat distribution is influenced by type of dietary fat fed to rats. Int J Obes Relat Metab Disord. 1993; 17(4):223–226. PMID: 8387971.
23. Kimura Y, Sumiyoshi M. Effects of various Eleutherococcus senticosus cortex on swimming time, natural killer activity and corticosterone level in forced swimming stressed mice. J Ethnopharmacol. 2004; 95(2-3):447–453. PMID: 15507373.

24. Cairns SP. Lactic acid and exercise performance: culprit or friend? Sports Med. 2006; 36(4):279–291. PMID: 16573355.
25. Robergs RA, Ghiasvand F, Parker D. Biochemistry of exerciseinduced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004; 287(3):R502–R516. PMID: 15308499.

26. Carvalho-Peixoto J, Alves RC, Cameron LC. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl Physiol Nutr Metab. 2007; 32(6):1186–1190. PMID: 18059593.

27. Nybo L, Dalsgaard MK, Steensberg A, Møller K, Secher NH. Cerebral ammonia uptake and accumulation during prolonged exercise in humans. J Physiol. 2005; 563:285–290. PMID: 15611036.

28. Kimber NE, Heigenhauser GJ, Spriet LL, Dyck DJ. Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. J Physiol. 2003; 548:919–927. PMID: 12651914.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download