Abstract
The sheep spine is widely used as a model for preclinical research in human medicine to test new spinal implants and surgical procedures. Therefore, precise morphometric data are needed. The present study aimed to provide computed tomographic (CT) morphometry of sheep thoracolumbar spine. Five adult normal Merino sheep were included in this study. Sheep were anaesthetised and positioned in sternal recumbency. Subsequently, transverse and sagittal images were obtained using a multi-detector-row helical CT scanner. Measurements of the vertebral bodies, pedicles, intervertebral disc and transverse processes were performed with dedicated software. Vertebral bodies and the spinal canal were wider than they were deep, most obviously in the lumbar vertebrae. The intervertebral discs were as much as 57.4% thicker in the lumbar than in the thoracic spine. The pedicles were higher and longer than they were wide over the entire thoracolumbar spine. In conclusion, the generated data can serve as a CT reference for the ovine thoracolumbar spine and may be helpful in using sheep spine as a model for human spinal research.
In vitro experiments are useful in providing basic understanding of the biomechanical and functional features of the spine, and thus more insight into the physiological and pathological functions. Furthermore, new spinal implants and surgical procedures are often tested pre-clinically on cadaver spines [1,2].
Human specimens are preferable for these models because they mimic the physiological situation as much as possible. However, there are some difficulties in using the human model, such as obtaining it fresh especially from a healthy population and in large quantities in order to obviate the wide scattering effect associated with biological variability [3]. Moreover, in vitro studies do not provide time-dependent changes of biomechanics, histological and functional behaviour after applying instruments [4]. Therefore, animal models represent a suitable alternative, these being available and having more uniform geometrical and mechanical properties than humans when selected for breed, age and weight [5-8].
To mimic the human spine, an appropriate animal should be used which has biomechanical characteristics and anatomical dimensions of the spine as similar as possible to those in humans. Furthermore, precise geometrical data of animal models are needed for mathematical models [9,10]. The sheep spine is frequently used as a model for human spinal orthopaedic researches and is well accepted due to similarities with humans in weight, bone and joint structure and the bone remodelling process [11-14] Moreover, sheep are easily available, inexpensive, easy to handle and well accepted as an ethical animal model [15].
Computed tomography (CT) is a non-invasive imaging modality which has been used extensively in human to perform in vivo morphometric analysis of the spine [16,17] and describe the normal variation in size and shape of the human vertebrae at various spinal levels [18-20].
Measurement accuracy represents the core of morphometric studies. Therefore, the factors affecting the accuracy should be addressed. The accuracy of the measurements based on CT images is affected by scanning parameters [21] and viewer control setting [22].
Morphometry of sheep thoracolumbar spine is essential for the design and interpretation of results derived from studies which contemplate their use. This study aims to provide quantitative reference values of healthy ovine thoracolumbar using CT.
To reduce the numbers of animals, 5 female Merino sheep without any history or clinical signs related to spinal diseases were included. The mean age of the sheep was 2.0±0.1 year. Mean body weight was 62.0±5.3 kg. The study was approved by the Animal Protection Agency regional office Leipzig.
Each sheep was fasted for 24 hours and deprived of water for 12 hours before being premedicated with 0.1 mg/kg atropine sulphate (Atropinum sulfuricum 0.5 mg Eifelango®, Eifelango, Bad Neuenahr-Ahrweiler, Germany), and a combination of 0.1 mg/kg butorphanol tartrate (Alvegesic®, CP-Pharma GmbH, Burgdorf, Germany) and 0.2 mg/kg midazolam (Midazolam, B. Braun; B. Braun, Melsung, Germany) administered intravenously. Anaesthesia was induced with 3 mg/kg ketamine chlorhydrate (Ursotamin®, Serumwerk Bernburg AG, Bernburg, Germany) intravenously. After endotracheal intubation, anaesthesia was maintained with isoflurane (Isofluran CP®, CP-Pharma GmbH) delivered in oxygen through an endotracheal tube.
The sheep were positioned in sternal recumbency and intravenous fluid bags were used to obtain a perpendicular position of the spine relative to the x-ray beam of the gantry. Contiguous slices were obtained from the cranial aspect of T2 to the caudal aspect of L6 with a multi-detector-row helical CT unit (Philips Medical Systems MX8000 IDT 16, Hamburg, Germany). Technical settings were 120 kV, 200 mA, 0.75 second tube rotation and a pitch of 0.438. The data were reconstructed to a transverse and sagittal image series with slice thickness ranging between 0.3-1.2 mm using a high-frequency image reconstruction algorithm (bone). Window width and level settings were standardised for all measurements (window width, 2000 Hounsfield units; window level, 500 Hounsfield units). The CT images were reconstructed using multi planar reconstruction in transverse and sagittlal planes. Transverse images were reconstructed parallel to the cranial endplate of the vertebral body, whereas the sagittal images were reconstructed at two levels. The first level was at the midsagittal plane of the vertebra to measure some of the vertebral body and intervertebral disc dimensions. The second level was at the midsagittal plane of the left or right pedicle for measuring the pedicle height, whereby we assumed there was no difference between the left and right pedicle. Subsequently, CT images were transferred to a work station and reviewed with dedicated software (CuraSmartClient curasystems GmbH, Ettlingen, Germany). From the transverse images series, a single CT image through the mid level of the cranial third of the pedicle was selected for measuring. This level demonstrates individual features of each vertebra relative to adjacent vertebra.
Eleven parameters were measured from the transverse images and four parameters from the sagittal images for each spinal level (Table 1, Figure 1-5). Parameters of the vertebral body, spinal canal and transverse processes were measured as described in human literature [23]. The vertebral body measurements (Figure 1,2) included the distance between the lateral borders of the vertebral body in the transverse plane of the cranial endplate, termed the vertebral body width (VBW), and the distance between the dorsal and ventral borders of the vertebral body, termed the vertebral body depth (WBD). The distance between the cranial and caudal endplates of the vertebral body at the dorsal margin was measured from the sagittal image was termed the vertebral body height dorsal (VBHd). The same distance at the ventral margin was termed the vertebral body height ventral (VBHv). Cortical bone thickness (CBT) was assessed as the distance between the outer and inner borders of the lateral part of the vertebral body on the transverse image. Disc Thickness (DT) was measured at the middle level of the intervertebral disc on the sagittal image. In this study, DT refers to the disc which located cranial to the mentioned vertebral level. The spinal canal parameters included spinal canal width (SCW) and depth (SCD) and were measured on transverse images (Figure 3). SCW was measured as the distance between the axial pedicle cortices, while SCD was defined as the distance from the dorsal border of the vertebral body to the lamina at the midline. Transverse process length (TPL) was the distance between the tips of the transverse processes measured on the transverse image (Figure 3).
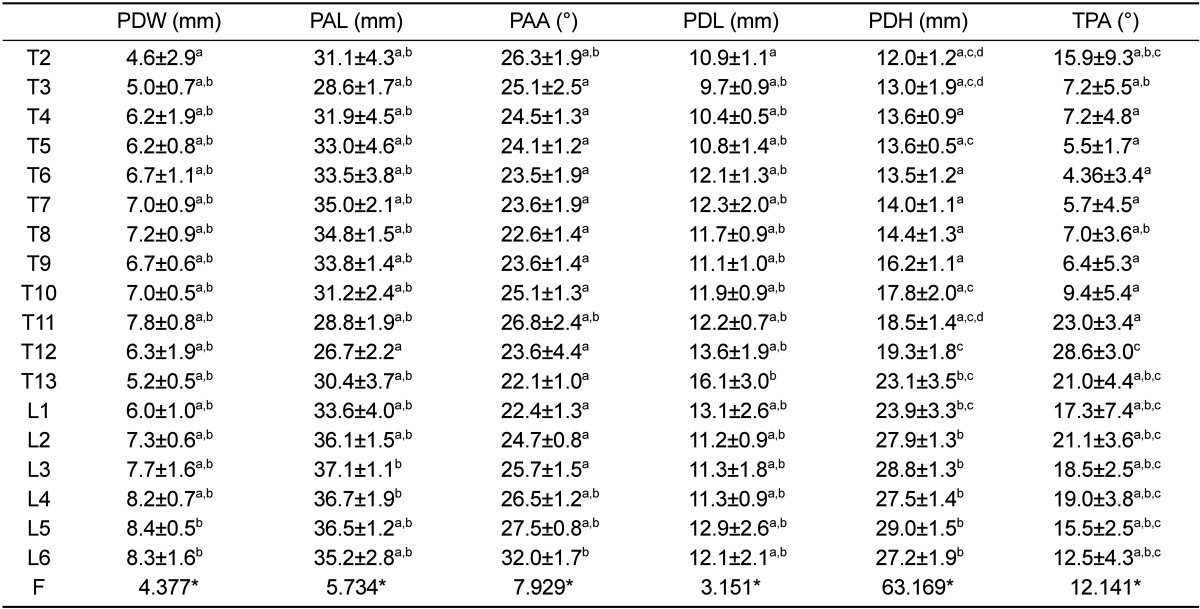
The pedicle width (PDW) was also measured on the transverse image of each vertebra as the narrowest part of the pedicle (Figure 4). The pedicle height (PDH) was measured on the sagittal image in the same manner as PDW. The pedicle axis length (PAL) was measured from the dorsal cortex of the articular facet to the midpoint of the ventral vertebral body cortex on the transverse plane, while the angle between PAL and the vertebra sagittal midline was defined as the pedicle axis angle (PAA) (Figure 4). According to the location of the pedicle to the transverse process, the vertebrae were divided into types I and II. In type I, the pedicles were located ventrally to the transverse process. The pedicle length (PDL) was therefore measured as a distance between the dorsal pedicle cortex and the perpendicular line to the vertebral midline, which is tangent to the ventral border of the spinal canal (Figure 5). In type II, the pedicles were located dorsally to the transverse process. Thus, the PDL was measured as a distance between the dorsal pedicle cortex and junction point of the ventral border of the transverse process and vertebral body (Figure 4). The angle between the line halvings the pedicle and vertebral sagittal midline in the transverse plane was termed transverse pedicle angle (TPA). Each parameter was measured six times by the same observer (MM).
Intra-observer reliability was calculated. For each sheep three vertebral levels per parameter were randomly selected to detect the intra-observer reliability which was represented by the coefficient of variation (CV). One-way analysis of variance and the Scheffe test were used to determine differences between the vertebral levels for each parameter. Commercially available software was used for statistical analysis (Microsoft Excel 2010, Microsoft Deutschland GmbH, Unterschleissheim, Germany). The level of significance was set at P<0.0001.
Repeated measurements of spinal parameters revealed a high level of reliability, where CV values of all parameters were less than 5% (Table 1). In Tables 2-4, mean and standard deviation of the CT measurements in thoracolumbar spines of the investigated 5 Merino sheep are presented.
The maximum value of VBHd was observed at L6 and the smallest at T3. VBHd was fairly constant at about 25 mm in the cranial thoracic region, then increasing steadily until L6. For the VBHv, the maximum value was found at the level of L6 and the smallest at the level of T7. At the level of T10, VBHd and VBHv had a similar value. In the lumbar region, VBHd became larger than VBHv, as much as 1.8 mm. DT ranged between 1.3 and 2.1 mm in the thoracic region, while in the lumbar region it showed greater values ranging between 2.6 and 3.3 mm. It was as much as 1.6 mm thicker in the lumbar than in the thoracic spine. Statistically significant differences (P<0.0001) were observed in VBHd, VBHv and DT between the vertebral levels from T2 to L6. At L6 level the maximum value of VBW was observed and the minimum at T8. The minimum value of WBD was found at T12 level and the maximum at T8. The vertebral body was wider than it was deep over the whole thoracolumbar spine, which was most obvious in the lumbar vertebrae. CBT showed the maximum value at T2 and the minimum at T5. There was no significant difference in VBW, VBD and CBT between the vertebral levels. The vertebral body and intervertebral disc measurements are listed in Table 2.
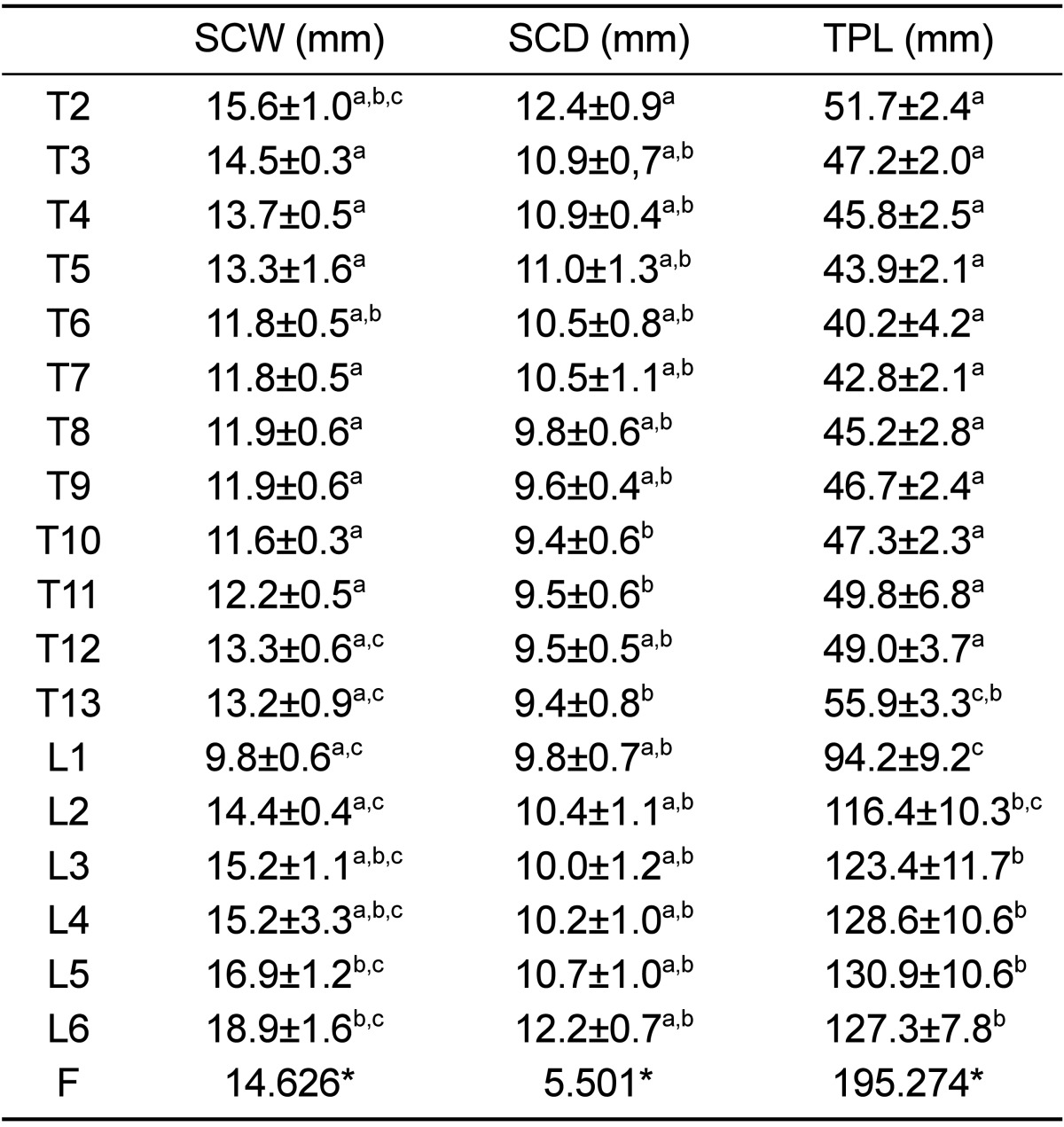
The spinal canal was the widest at T2 in the thoracic region, then narrowing at T10 and increasing again in the lumbar region to reach the maximum width over the entire thoracolumbar spine at L6. SCD showed the maximum value at T2; then decreasing slightly at T10, thereafter increasing at L6. In the lumbar region SCW was as much as 5.4 mm larger than SCD, while the mid thoracic region showed the smallest spinal canal dimensions over the entire thoracolumbar region. The spinal canal showed a trend similar to the vertebral body, which was wider than it was deep over the whole thoracolumbar spine. L1 was exceptionally different, the canal width and depth being equal. TPL decreased slightly from T2 to T12 and then reached the maximum value at level L5. The previous parameters are listed in Table 3. There were significant differences (P<0.0001) in previous parameters between the vertebral levels.
Pedicle parameters are shown in Table 4. PDW ranged from 4.6 to 8.4 mm and showed an increase from the cranial thoracic spine toward the caudal level lumbar vertebrae reaching the maximum value at L5. PDH showed the lowest value at T2, while the highest value was observed at L5. PAL ranged between 26.7 and 37.1 mm. T7 showed the maximum value in the thoracic region, then decreasing caudally at T12, which was the shortest length in the thoracolumbar spine, and increased again until L3. PAA decreased from T2 to T8, thereafter increasing until T11, and decreased again at level of T13. In the lumbar region, PAA showed a constant increase, reaching the maximum value in the whole of the thoracolumbar spine at L6. TPA was the greatest at T12 level, decreasing caudally to this level until L6, while the lowest value over the whole thoracolumbar spine was observed at level T6. However, a significant (P<0.0001) difference was observed in all pedicle parameters between the vertebral levels.
Human spines are difficult to obtain fresh and in large quantities for in vitro studies. Therefore, animal spines represent a suitable alternative. Sheep are claimed to be one of the most representative animal models for orthopaedic research [24] and precise morphometrical data are needed when sheep are used as a model for orthopaedic spinal research. The present study, therefore, provided CT reference values for the thoracolumbar spine of healthy sheep.
A variety of animal species have been used as model for human orthopaedic studies. Martini et al. [24] compared the sheep to the other available animal models for human orthopaedic researches. They reported nonhuman primates provide an excellent model thanks to their analogy with humans, but are not cost-efficient, require stringent controls and could cause severe zoonotic diseases, as well as the ethical pressures of using this species. In spite the physiological similarity between human and pigs there are some problems limiting their use such as rapid body growth and weight which affect the long term orthopaedic studies. Small pig breeds can be used to minimize the previous problems but they are more expensive and sometimes difficult to recruit [24]. Because of the previously mentioned considerations, sheep are becoming popular as animal models in orthopaedic research. Furthermore, sheep are quite similar in body weight to humans, and sufficiently large to allow serial sampling and multiple experimental procedures. Wilke et al. [28] compared the quantitative biomechanical properties of the sheep spine to human and concluded there is biomechanical similarities of sheep and human spines and the sheep spine can serve as model for the evaluation of spinal implants.
To obtain uniformity, the animals were of equal age, weight, sex and breed. The number of 5 animals used was the lowest possible to comply with the rules of 3R but sufficient enough to provide reliable data [5-8]. Sheep in the present study were female because osteoporosis studies are most commonly conducted on female gender.
It could be argued that the number of sheep spines used was small. In order to overcome the problem of a small sample size, significance for analysis was set using a low p value (P<0.0001). However, the nearly similar sheep dimensions and small variance around the mean indicated that a larger sample size was not necessary. Moreover, previous investigators had used comparable sample sizes for similar studies [25-29].
CT is the examination of choice for assessing the bony structures of the spine. The perceived image quality depends on the choice of imaging parameters and also on the post-processing, in particular the reconstruction algorithm and the reformatting parameters, as well as the mode of display [30].
Dorsal recumbency is the position of choice for spine CT imaging, because it ensures minimal respiratory movements of the spine. In our study, sheep were positioned in sternal recumbency for two reasons: Firstly, with sternal recumbency, we could mimic the natural position of the spine as much as possible, particularly the kyphosis in lumbar spine. Secondly, this was performed to minimise the complications of general anaesthesia [31]. Short tube rotation time (0.75 second) setting was used to minimise the influence of respiratory movements on image quality [32,33].
Slice thickness affects an image's quality through its influence on spatial resolution. However, thin slice thickness reduces the amount of volume averaging and thus improves spatial resolution. For orthopaedic imaging, scanning with thin-slice collimation is preferable, ideally 1.5 mm or less [30], Therefore, slice thickness in the current study was less than 1.5 mm. Decreasing slice thickness increases the image noise. To keep the noise at an acceptable level, high mA and wide window display should be used [32,33]. In the present study, therefore, CT scanning setting was 200 mA and 2000 Hounsfield units window width. These settings, moreover, are consistent with published spinal CT imaging protocol [33].
Pitch describes the relationship between the table increment during one full gantry rotation and the slice thickness [32,33]. Pitch is directly proportional to image blur. Therefore, a highly pitched CT scan results in a very blurry image. The pitch has to be less than 2 for orthopaedic imaging, which is often chosen significantly lower than this, around 0.3-0.5 for multi-slice CTs [30]. In this study, the pitch setting was within the aforementioned range (0.438).
The picture archiving and communication system instrumentation permits manipulation of the CT data, with adjustment of contrast for optimisation of image quality and measurement of distance, area and angle. Nevertheless, potential sources of error remain. One source of error is the accurate identification of precise anatomical points [23]. Intra-observer tests were carried out to analyse the magnitude of such errors. We found that the intra-observer error was within the limit of 5% [34]. Inter-observer error was not assessed, as all measurements for this database were performed by a single observer in order to maximise the CT measurement accuracy [22].
The results of the current study showed that the vertebral bodies were wider than deep, most obviously in the lumbar vertebrae. The spinal canal has a similar behaviour like vertebral bodies, while it tends to have nearly an equal width and depth at the caudal thoracic region. The intervertebral discs were thicker in the lumbar than in the thoracic spine. The pedicles were higher and longer than they were wide over the entire thoracolumbar spine.
We are aware of the elaborate work done by Wilke and co-workers [29]. They studied the anatomical dimensions of the vertebral body, pedicle, spinal canal, spinous and transverse processes, and articular facet and intervertebral disc for comparison with human data. Their database provides information regarding the anatomy of three- to four-year-old sheep.
There is agreement between their results and our findings. However, the measurements of the previous study tended to be 1.4-5.9 times larger than the present study. The causes of the difference could be attributed to age variation, as we used a 2-year-old sheep, and measuring methods. Wilke et al. [29] used a manual measurement method on the cadaveric spine, while we used CT. The manual measurements based on Vernier caliper were rounded to the nearest millimetre, which represents a potential measurement error of the order of 6-7% in measurements. Moreover, the irregular shape of the bony surfaces may induce some variability and or error when determining the dimensions of the vertebra [35,36].
In contrast to a previous study [29] the current study was carried out on live subjects and thus the influences of preservation methods on actual dimensions were excluded. Some parameters, such as PDL, PAL and PAA, have been reported here for the first time in sheep thoracolumbar spine. Therefore, it should be considered inevitable that sheep are used as a model for spinal fixation research [16].
The comparative biomechanical characteristics of the sheep were presented elsewhere [28]. We did not test the biomechanical properties of sheep spinal segments as this was not the aim of our study. However, the current results could be interpreted from a biomechanical perspective.
A previous study carried out on ovine spine stated that the dorsoventral movement was the highest at the L6 level [28]. The vertebral body shape has an influence on the spinal movements. However, the horizontal oval shape of the vertebral body facilitates the dorsoventral movements [37]. In our study L6 had the most horizontal oval vertebral body over the whole of the thoracolumbar spine, which can explain the observation of the highest range of dorsoventral movement at this level.
In humans, spinal canal dimensions have an influence on spine dorsoventral movement, where greater spinal canal dimensions facilitate the flexion motion [38,39]. In the current study, the spinal canal dimensions were the greatest at L6 and the lowest at T10. Based on these results, we expect the highest flexion to be at L6 and the lowest at T10. A biomechanical study carried out on sheep spine revealed that flexion was the highest at the L6 level (5.29°±0.82) and the lowest at T10 (1.93°±0.3) [28], which confirms our expectations.
In humans, the pedicle represents a stronger site for screw placement than the vertebral body. The trabeculae in the pedicle appear to be thicker and stronger. Moreover, the pedicle cortex is thicker allowing the screw threads to engage with cortical bone [16]. Pedicle morphometry plays an important role in transpedicular screw fixation, because it is related to screw placement [16]. The diameter of the screw should be 80% or less of the diameter of the pedicle [23]. Therefore, the current study presents the needed precise morphometrical data for using sheep as a model for transpedicular fixation research.
It was interesting to note that the PDW increased from cranial thoracic spine to caudal level lumbar vertebrae. PDW determines the diameter of appropriate transpedicular screws, the wider pedicle allowing the use of a thicker screw, which provides greater fixation.
No previous studies quantitatively measured PAL, PDL and PAA in the thoracolumbar spine of sheep, knowledge of which is important for transpedicular screw placement and prevention of perforation of the ventral aspect of the cortex by the screws and injury to vital structures.
PDL and PAL defined the minimum and maximum length of screw needed to obtain a grip on the entire pedicle, respectively. In our study, PDL was as much as 29.2-53.0% of PAL. Thus, a transpedicular screw length should be at least 53.0% of PAL. PAA may be an important parameter for correct pedicle screw placement. In humans, Louis [40] and Roy-Camille et al. [41] recommend that a pedicle screw should be inserted in a straight (vertical) direction. In contrast, Krag et al. [16] and Zindrick et al. [42] believe that insertion of the pedicle along the medial trajectory is a safer technique. Jahng et al. [43] reported in their experiments on sheep lumbar spine that there is a noticeable difference between the TPA and PAA, which is consistent with the current results. The difference between the TPA and PAA is most likely due to the different vertebral types (type I or II). Therefore, we predict a high misplacement rate when a pedicle screw is inserted in a straight direction.
In the lumbar region, DT was as much as 57.4% thicker than those in the thoracic vertebrae. A thicker disc provides more mobility than a thinner one [44]. In contrast, transverse processes were longer in the lumbar than in the thoracic region, which can explain the restriction of lateral bending and axial rotation in the lumbar compared to the thoracic region [28].
A limitation of this study could be the accuracy of the small measurement such as thickness of the cortical bone (less than 2 mm). This questionable, due to the influence of the volume averaging artefact. Therefore, a thin slice thickness was set to minimise the volume averaging effect.
In conclusion, this study provided a comprehensive quantitative database of the normal sheep thoracolumbar spine. This descriptive information can be used to help determine whether the sheep spine can be a representative model for testing a certain application. When testing new implants and surgical techniques, i.e. intrapedicular screw, scaling differences should be taken into account to select the suitable implants' size for application.
References
1. Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998; 7(2):148–154. PMID: 9629939.

2. Goel VK, Panjabi MM, Patwardhan AG, Dooris AP, Serhan H. American Society for Testing and Materials. Test protocols for evaluation of spinal implants. J Bone Joint Surg Am. 2006; 88(Suppl 2):103–109. PMID: 16595454.

3. Ashman RB, Bechtold JE, Edwards WT, Johnston CE 2nd, McAfee PC, Tencer AF. In vitro spinal arthrodesis implant mechanical testing protocols. J Spinal Disord. 1989; 2(4):274–281. PMID: 2520086.

4. Tominaga T, Dickman CA, Sonntag VK, Coons S. Comparative anatomy of the baboon and the human cervical spine. Spine. 1995; 20(2):131–137. PMID: 7716616.

5. Smit TH. The use of a quadruped as an in vivo model for the study of the spine - biomechanical considerations. Eur Spine J. 2002; 11(2):137–144. PMID: 11956920.

6. Edmondston SJ, Singer KP, Day RE, Breidahl PD, Price RI. Formalin fixation effects on vertebral bone density and failure mechanics: an in-vitro study of human and sheep vertebrae. Clin Biomech (Bristol, Avon). 1994; 9(3):175–179.

7. Eggli S, Schläpfer F, Angst M, Witschger P, Aebi M. Biomechanical testing of three newly developed transpedicular multisegmental fixation systems. Eur Spine J. 1992; 1(2):109–116. PMID: 20054957.

8. Gurwitz GS, Dawson JM, McNamara MJ, Federspiel CF, Spengler DM. Biomechanical analysis of three surgical approaches for lumbar burst fractures using short-segment instrumentation. Spine. 1993; 18(8):977–982. PMID: 8367785.

9. Yoganandan N, Kumaresan S, Voo L, Pintar FA. Finite element applications in human cervical spine modeling. Spine (Phila Pa 1976). 1996; 21(15):1824–1834. PMID: 8855470.

10. Kiefer A, Shirazi-Adl A, Parnianpour M. Stability of the human spine in neutral postures. Eur Spine J. 1997; 6(1):45–53. PMID: 9093827.

11. Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995; 16(4):277S–284S. PMID: 7626315.

12. Nunamaker DM. Experimental models of fracture repair. Clin Orthop Relat Res. 1998; 355:S56–S65. PMID: 9917626.

13. Bergmann G, Graichen F, Rohlmann A. Hip joint forces in sheep. J Biomech. 1999; 32(8):769–777. PMID: 10433418.

14. Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporos Int. 2005; 16:S129–S138. PMID: 15750681.

15. Turner AS. Experiences with sheep as an animal model for shoulder surgery: strengths and shortcomings. J Shoulder Elbow Surg. 2007; 16(5):S158–S163. PMID: 17507248.

16. Krag MH, Weaver DL, Beynnon BD, Haugh LD. Morphometry of the thoracic and lumbar spine related to transpedicular screw placement for surgical spinal fixation. Spine. 1988; 13(1):27–32. PMID: 3381134.

17. Olsewski JM, Simmons EH, Kallen FC, Mendel FC, Severin CM, Berens DL. Morphometry of the lumbar spine: anatomical perspectives related to transpedicular fixation. J Bone Joint Surg Am. 1990; 72(4):541–549. PMID: 2139030.
18. Abuzayed B, Tutunculer B, Kucukyuruk B, Tuzgen S. Anatomic basis of anterior and posterior instrumentation of the spine: morphometric study. Surg Radiol Anat. 2010; 32(1):75–85. PMID: 19696959.

19. Kadioglu HH, Takci E, Levent A, Arik M, Aydin IH. Measurements of the lumbar pedicles in the Eastern Anatolian population. Surg Radiol Anat. 2003; 25(2):120–126. PMID: 12748815.

20. Wolf A, Shoham M, Michael S, Moshe R. Morphometric study of the human lumbar spine for operation-workspace specifications. Spine. 2001; 26(22):2472–2477. PMID: 11707713.

21. Way TW, Chan HP, Goodsitt MM, Sahiner B, Hadjiiski LM, Zhou C, Chughtai A. Effect of CT scanning parameters on volumetric measurements of pulmonary nodules by 3D active contour segmentation: a phantom study. Phys Med Biol. 2008; 53(5):1295–1312. PMID: 18296763.

22. Beers GJ, Carter AP, Leiter BE, Tilak SP, Shah RR. Interobserver discrepancies in distance measurements from lumbar spine CT scans. Am J Roentgenol. 1985; 144(2):395–398. PMID: 3871289.

23. Zhou SH, McCarthy ID, McGregor AH, Coombs RR, Hughes SP. Geometrical dimensions of the lower lumbar vertebrae--analysis of data from digitised CT images. Eur Spine J. 2000; 9(3):242–248. PMID: 10905444.
24. Martini L, Fini M, Giavaresi G, Giardino R. Sheep model in orthopedic research: a literature review. Comp Med. 2001; 51(4):292–299. PMID: 11924786.
25. Kumar N, Kukreti S, Ishaque M, Mulholland R. Anatomy of deer spine and its comparison to the human spine. Anat Rec. 2000; 260(2):189–203. PMID: 10993955.

26. McLain RF, Yerby SA, Moseley TA. Comparative morphometry of L4 vertebrae: comparison of large animal models for the human lumbar spine. Spine (Phila Pa 1976). 2002; 27(8):E200–E206. PMID: 11935119.
27. Riley LH 3rd, Eck JC, Yoshida H, Koh YD, You JW, Lim TH. A biomechanical comparison of calf versus cadaver lumbar spine models. Spine. 2004; 29(11):E217–E220. PMID: 15167671.

28. Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997; 22(20):2365–2374. PMID: 9355217.

29. Wilke HJ, Kettler A, Wenger KH, Claes LE. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997; 247(4):542–555. PMID: 9096794.

30. Tins B. Technical aspects of CT imaging of the spine. Insights imaging. 2010; 1(5-6):349–359. PMID: 22347928.

31. Mitchell B, Williams J. Respiratory function changes in sheep associated with lying in lateral recumbency and with sedation by xylazine. Vet Anaesth Analg. 1976; 6(1):30–36.

32. Schwarz T, Saunders J. CT acquisitation principle. In : Schwarz T, Saunders J, editors. Veterinary computed tomography. 1st ed. Oxford: Wiley-Blackwell;2011. p. 9–27.
33. Seiler G, Kinns J, Dennison S, Saunders J, Schwarz T. Vertebral column and spinal cord. In : Schwarz T, Saunders J, editors. Veterinary computed tomography. 1st ed. Oxford: Wiley-Blackwell;2011. p. 209–228.
34. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1(8476):307–310. PMID: 2868172.

35. Flynn JR, Bolton PS. Measurement of the vertebral canal dimensions of the neck of the rat with a comparison to the human. Anat Rec. 2007; 290(7):893–899.

36. Tatarek NE. Variation in the human cervical neural canal. Spine J. 2005; 5(6):623–631. PMID: 16291101.

37. Denoix JM. Spinal biomechanics and functional anatomy. Vet Clin North Am Equine Pract. 1999; 15(1):27–60. PMID: 10218240.

38. Schönström N, Lindahl S, Willén J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res. 1989; 7(1):115–121. PMID: 2908901.

39. Inufusa A, An HS, Lim TH, Hasegawa T, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996; 21(21):2412–2420. PMID: 8923625.

40. Louis R. Fusion of the lumbar and sacral spine by internal fixation with screw plates. Clin Orthop Relat Res. 1986; 203:18–33. PMID: 3955980.

41. Roy-Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop Relat Res. 1986; 203:7–17. PMID: 3955999.

42. Zindrick MR, Wiltse LL, Doornik A, Widell EH, Knight GW, Patwardhan AG, Thomas JC, Rothman SL, Fields BT. Analysis of the morphometric characteristics of the thoracic and lumbar pedicles. Spine. 1987; 12(2):160–166. PMID: 3589807.

43. Jahng TA, Fu TS, Kim DH. Open versus endoscopic lumbar pedicle screw fixation and posterolateral fusion in a sheep model: a feasibility study. Spine J. 2004; 4(5):519–526. PMID: 15363422.

44. Haussler KK. Anatomy of the thoracolumbar vertebral region. Vet Clin North Am Equine Pract. 1999; 15(1):13–26. PMID: 10218239.

Figure 1
Transverse CT image obtained at the cranial aspect of L5 of a 2-year-old clinically normal female Merino sheep illustrating the measurements obtained for T2 through L6. Left is right. The measurements of interest obtained for each of the thoracolumbar vertebrae were vertebral body width (VBW; widest distance between the lateral borders of the vertebral body), and vertebral body depth (VBD; distance between dorsal and ventral borders of vertebral body).
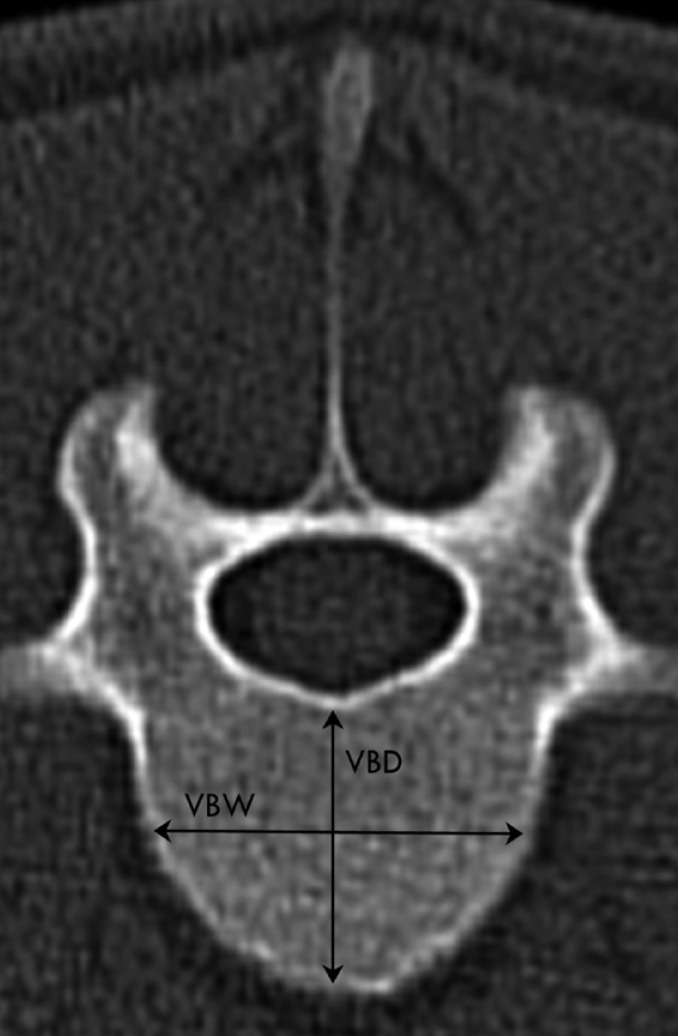
Figure 2
Sagittal CT image with measurements on the T9 in a two-year-old female Merino sheep illustrating vertebral body height at dorsal border (VBHd; distance between the most dorsocranial and the most dorsocaudal point of the same vertebral body), vertebral body height at ventral border (VBHv; distance between the most ventrocranial and the most ventrocaudal point of the same vertebral body) and disc thickness (DT; distance between cranial and caudal vertebral epiphyses of adjacent vertebrae). Cranial is to the left.
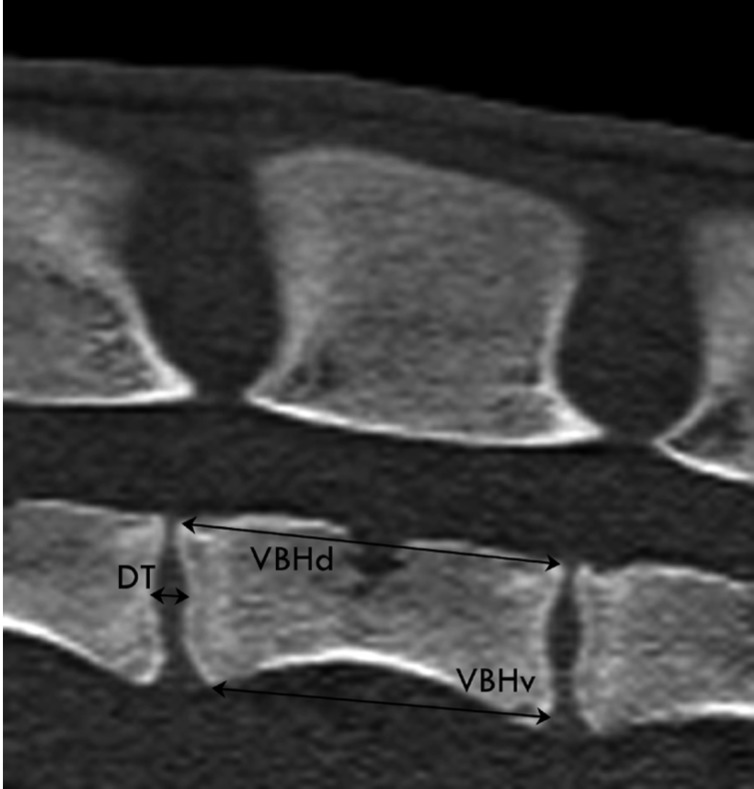
Figure 3
Transverse CT images obtained at the level of T7 of the same sheep as in Figure 1. Left is right. Spinal canal width (SCW; widest distance between axial cortices of pedicles), spinal canal depth (SCD; distance between dorsal border of vertebral body and lamina at vertebrae midline) and transverse process length (TPL; distance between tips of transverse processes).
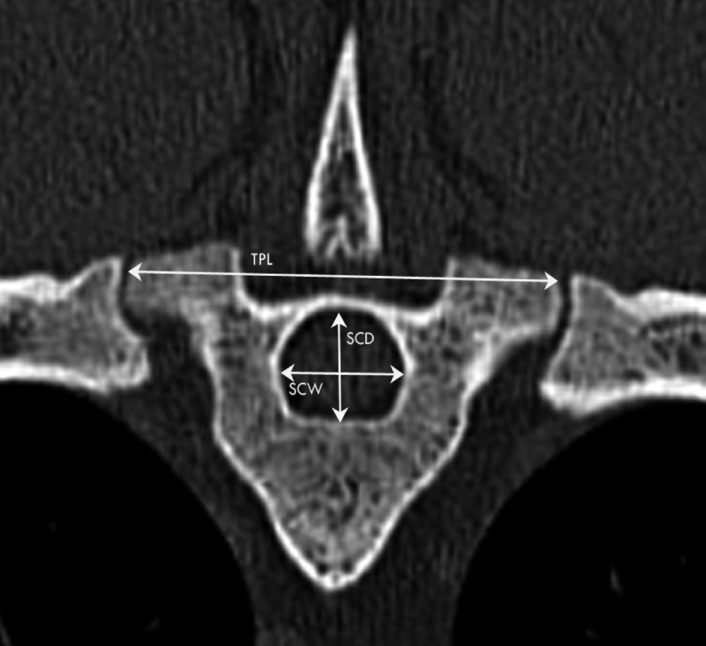
Figure 4
Transverse CT image obtained at the cranial aspect of L5 in a two-year-old female Merino sheep. Left is right. Pedicle length (PDL; distance between dorsal vertebral cortex and junction between ventral border of transverse process and vertebral body because of the vertebrae type II [the pedicle locates dorsal to the transverse process]),pedicle width (PDW;widest distance between the axial and abaxial border of pedicle), pedicle axis length (PAL; distance from dorsal vertebral lamina cortex to midpoint of ventral vertebral cortex, pedicle axis angle (PAA; angle between PAL and vertebral midline) and sagittal midline (ML; line bisects the vertebrae to equal halves).
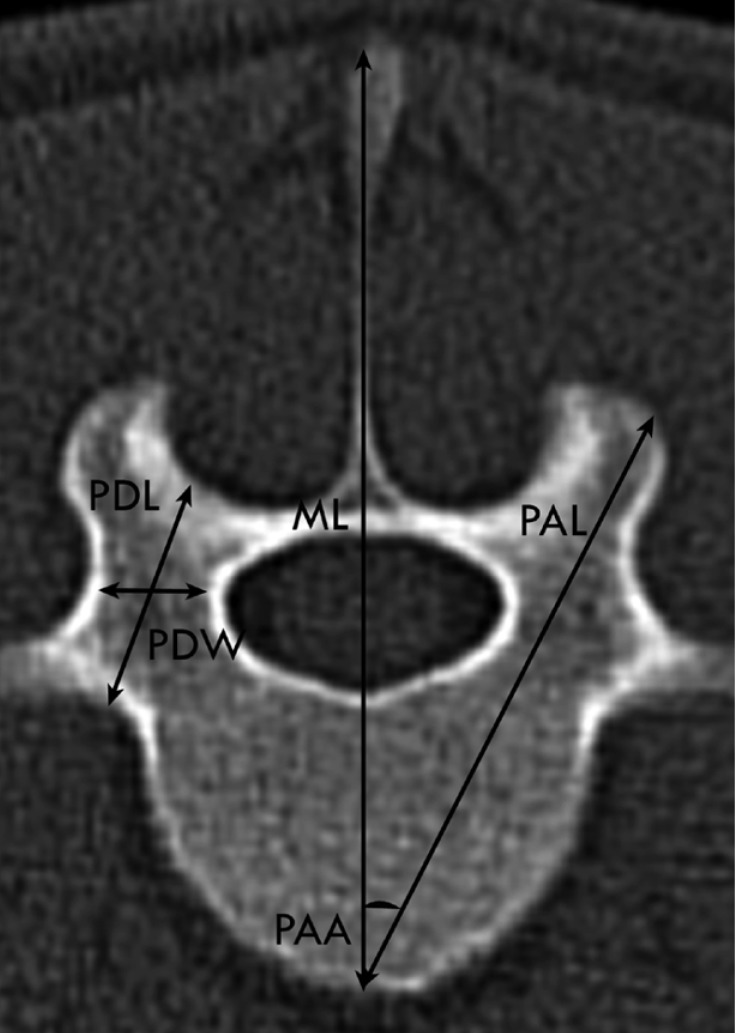
Figure 5
Transverse CT image obtained at the level of T5 in a two-year-old female Merino sheep. Left is right. Pedicle length (PDL; refers to pedicle type I, which is located ventrally to the transverse process), sagittal midline (ML; line bisects the vertebrae to equal halves) and PL (perpendicular line to vertebral midline at the level of ventral border of the spinal canal).
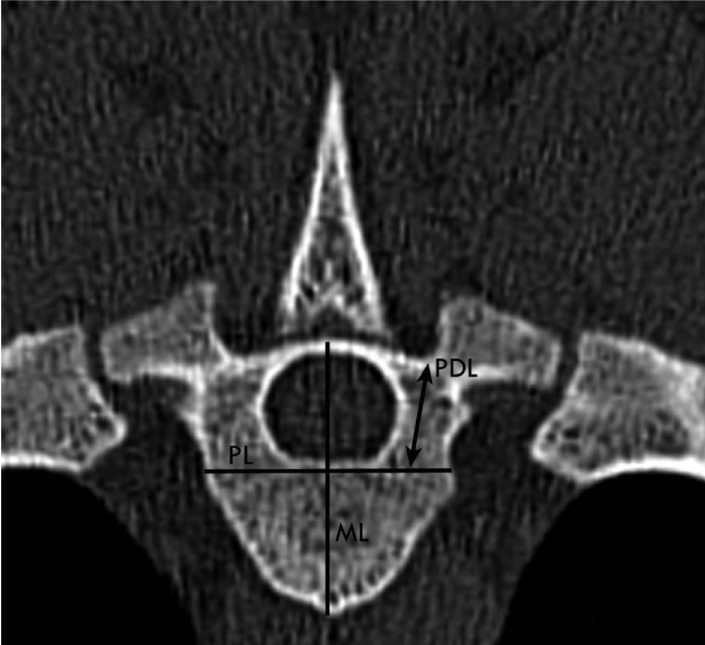
Table 1
Mean of Coefficient of variation (CV) values of thoracolumbar spine measurements of healthy Merino-sheep
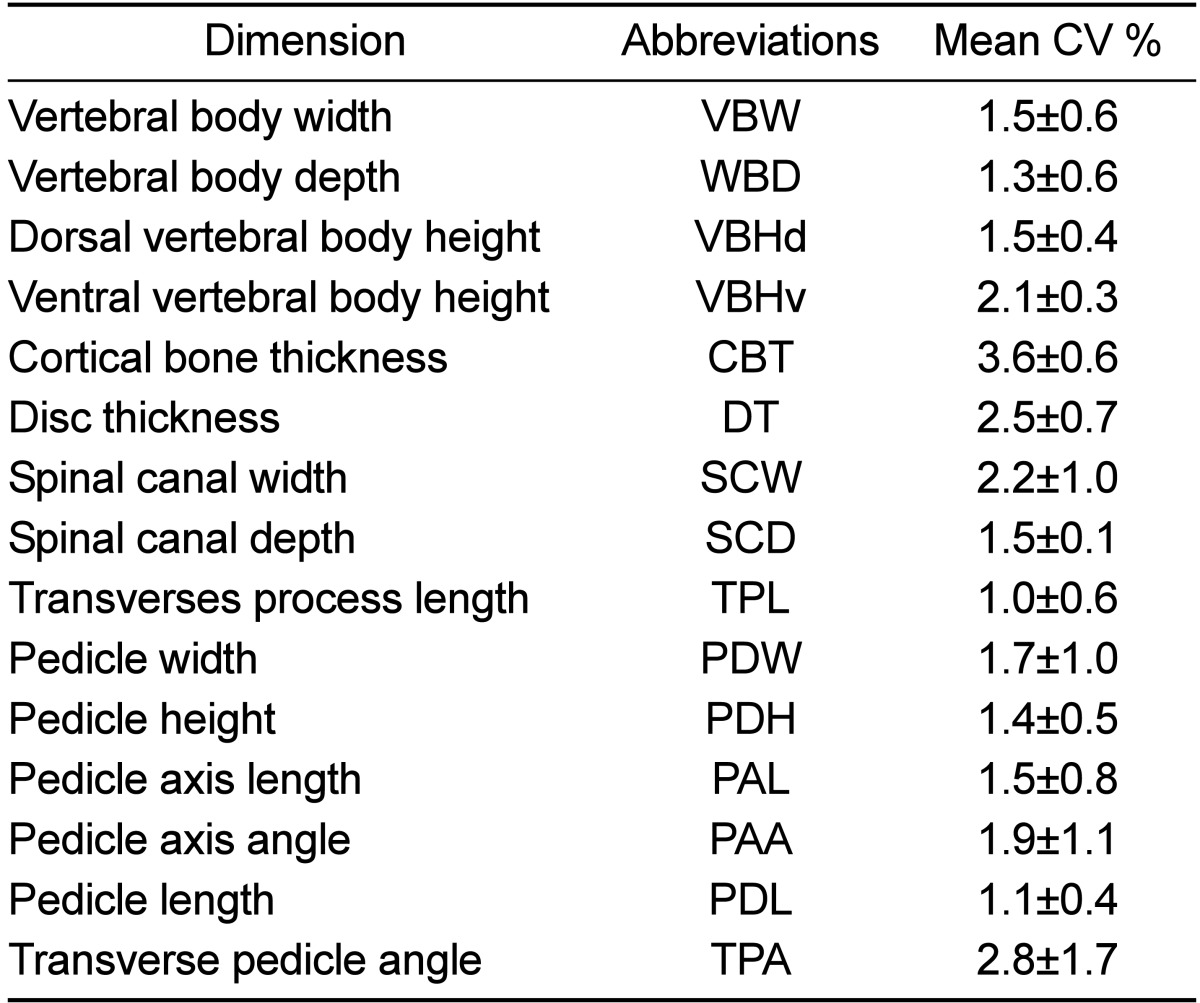
Table 2
Mean and standard deviation of CT measurements dimensions related to intervertebral disc and vertebral bodies of thoracolumbar spine of healthy Merino-sheep
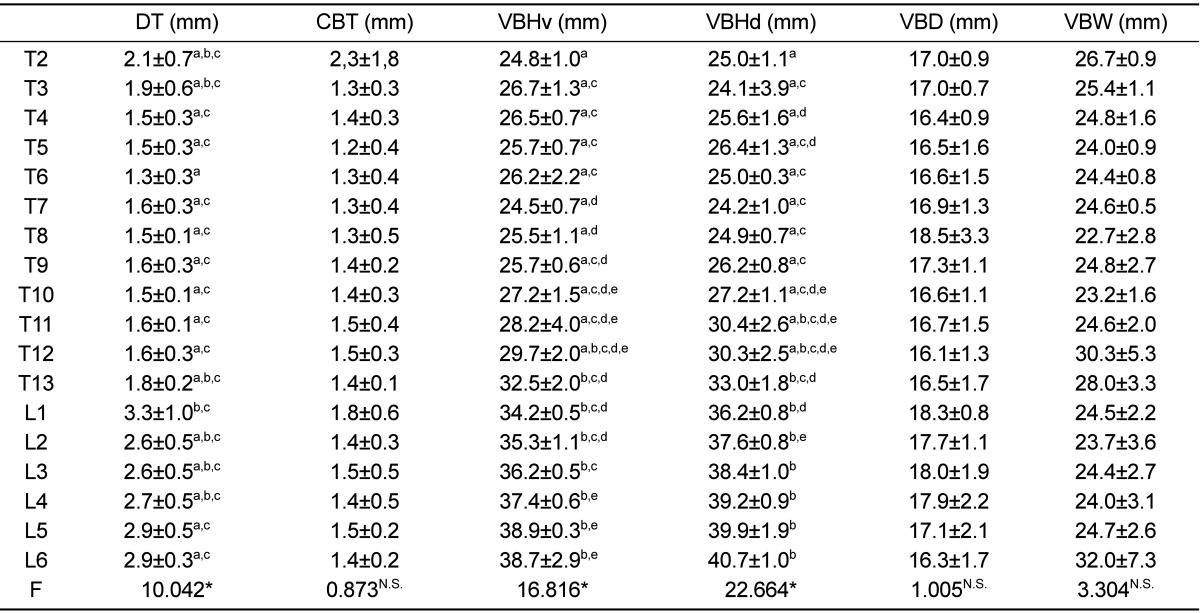
Table 3
Mean and standard deviation of CT measurements dimensions related to spinal canal and transverse processes of thoracolumbar spine of healthy Merino-sheep




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download