Abstract
Atopic dermatitis (AD), which is known as the most common pruritic skin disease, is caused by epidermal barrier dysfunction, allergies, microwave radiation, histamine intolerance, and genetic defects. To investigate the therapeutic effects of fermented soycrud (FSC) on AD pathology, alteration of AD phenotypes induced by phthalic anhydride (PA) treatment was assessed by ear thickness analysis, measurement of immune-related organ weights, ELISA, and histological and pathological analyses of ICR mice after FSC treatment for 2 weeks. Except for water content, the concentrations of most major components were lower in FSC compared to common tofu (CMT). Thymus and lymph node weights were significantly reduced in ICR mice treated with PA+CMT or PA+FSC, whereas spleen and body weights were maintained. Elevation of ear thickness induced by PA treatment was rapidly diminished in the CMT- and FSC-treated groups, although there was no significant difference between the two groups. Furthermore, significant reduction of epidermal thickness was detected in both the PA+CMT- and PA+FSC-treated groups. However, IgE concentration and dermal thickness were reduced only by PA+FSC treatment, whereas PA+CMT treatment maintained levels comparable to PA+vehicle treatment. The number of infiltrated mast cells was higher in the PA+vehicle-treated group compared to the untreated control. Following CMT or FSC treatment, mast cell infiltration was slightly reduced, although the CMT-treated group showed greater cell numbers. These results indicate that FSC may significantly relieve the phenotypes of AD induced by PA treatment and should be considered as a potential candidate for AD therapy.
AD is a common chronic inflammatory disorder caused by excessive activation of white blood cells and basophils due to IgE production in response to environmental antigens [1,2]. Further, this disorder is characterized by eczematous skin lesions, which give rise to asthma, allergic rhinitis, food allergies, and contact dermatitis [3,4]. Risk factors of AD include four major environmental candidates, including infectious disease, environmental pollution, exposure to allergens, and dietary changes [5].
Various medications, including topical corticosteroids, calcineurin inhibitors, antihistamines, oral corticosteroids, cyclosporine, and interferon, are commonly used for the treatment of AD, although their usage is limited due to undesirable side effects [6,7]. Especially, topical corticosteroids are the most common and effective treatment for AD in most countries as they can be directly applied to the skin as a cream or ointment [8]. Although they are effective on thin and sensitive skin areas, their application is problematic due to adverse events, such as skin thinning, and their potential to impair the skin barrier. However, topical calcineurin inhibitors also provide effective AD treatment without impairing the skin barrier or inducing skin thinning, although they are associated with a higher incidence of application-site reactions involving pruritus and skin-burning [9]. Recently, various foods and food derivatives have received attention as novel therapeutic drugs for AD treatment.
Tofu, also called bean curd, is an unfermented soybean product made by the coagulation of soy juice. It has a low calorie count, relatively large amount of protein, little fat content, and high concentrations of iron, calcium, and magnesium [10,11]. Especially, tofu is rich in isoflavones (509 µg/g), which have been suggested to be preventive against various cancers [12]. Until now, some studies have reported the beneficial effects of tofu in mammals. Tofu diets are known to exhibit therapeutic properties based on their ability to reduce certain lipid components, including cholesterol, triglycerides, and low-density lipoprotein (LDL), in the liver and serum [13-15]. Further, consumption of tofu can have preventive effects against several types of cancers, including prostate cancer, breast cancer, stomach cancer, and acute leukemia [16-19]. Tofu diets containing high concentrations of isoflavones (150-250 ppm) show elevated superoxide dismutase and catalase activities in various organs of SD rats [20]. However, there has been no report on whether or not fermented soycrud (FSC) can improve the pathological symptoms of AD.
Accordingly, in this study, we measured the benefit effects of FSC in a chemically induced AD model showing significant increases in ear thickness, IgE concentration, immune related organ weights, and mast cell infiltration. These results are the first to provide evidence that FSC may contribute to the relief of AD pathogenesis.
All animal experimental procedures performed in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Pusan National University (PNU-2012-0061). The animals were handled at the Pusan National University-Laboratory Animal Resources Center accredited by the Korea FDA in accordance with USA NIH guidelines (Accredited Unit Number-00231). All mice were housed under specified pathogen-free (SPF) conditions and a strict light cycle (lights on at 06:00 h and off at 18:00 h) and provided a standard irradiated chow diet (Purina Mills Inc., Missouri, USA) ad libitum. ICR mice used in this study were purchased from SamTako Biokorea (Osan, Korea).
Soybeans (Dae pung, 100 g) were soaked in distilled water (800 mL) at 27-32℃ for 6 h and then ground in a grinder with water. To synthesize FSC, soymilk was subsequently sterilized at 121℃ for 15 min in an autoclave machine. After cooling at room temperature, the soymilk was inoculated with Leuconostoc mesenteroides No. 4395 to a final concentration of 2%, followed by incubation at 30℃ for 24 h. Meanwhile, CMT produced by a traditional method was purchased from Pulmuone co. (Seoul, Korea) and then diluted with dH2O. CMT and FSC samples were freeze-dried and homogenized, after which both powders were stored at -75℃ before use.
Amounts of water, ash, crude protein, crude fat, and crude fiber in soycurd were determined according to the methods of Association of Official Analytical Chemists (AOAC) (1). Contents of total and reducing sugars were measured by the phenol-sulfuric acid [21] and 3,5-dinitrosalicylicacid (DNS) [22] methods, respectively.
Six-week-old ICR mice (n=21) were randomly divided into four groups. In the first group of ICR mice (No-treated, n=5), nothing was spread on the dorsum of ears for 2 weeks. In the second group (PA, n=16), 100 µL of 5% PA solution in AOO (4:1 acetone-olive oil, v/v) was repeatedly spread on the dorsum of ears daily for 2 weeks. The second group was further divided into three treatment groups: PA+vehicle, PA+CMT, and PA+FSC. The PA+CMT and PA+FSC groups received 0.3 mg of CMT and FSC powder, respectively, daily via oral administration for 2 weeks, whereas the PA+vehicle group received a comparable volume of water.
Alteration of body weight during the experimental procedure was measured daily for 2 weeks using an electronic balance (Mettler Toledo, Greifensee, Switzerland). The weights of three organs, including the thymus, spleen, and lymph nodes, collected from sacrificed mice were also measured by the same method. Ear thickness was measured using a thickness gauge (Digimatic Indicator, Matusutoyo Co., Tokyo, Japan) in order to determine the degree of allergic skin inflammation induced by PA treatment.
Serum IgE concentration was measured using an ELISA kit (Shibayagi, Inc., Gunma, Japan) according to the manufacturer's instructions. Briefly, capture antibodies were plated on the Nunc C bottom immunoplate supplied in the kit, after which the wells were washed with washing solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0) three times. Serum samples and standards diluted with buffer solution were then added to each of the wells, followed by incubation for 2 h. After the wells were washed again with washing solution, 50 µL of Biotin-conjugated anti-IgE antibody (1,000-fold dilution) was added to each well and the plate incubated for 2 h in order to facilitate binding with captured IgE. The wells were washed a final time with washing solution, after which horseradish peroxidase-conjugated detection antibody (2,000-fold dilution) was added to each well and the plate incubated for 1 h. An enzyme reaction was then initiated by addition of tetramethyl-benzidine (TMB) substrate solution (100 mM sodium acetate buffer, pH 6.0, 0.006% H2O2), followed by incubation at room temperature in the dark for 20 min. Finally, the reaction was terminated by addition of acidic solution (reaction stopper, 1M H2SO4), and the absorbance of yellow product was measured spectrophotometrically at 450 nm. The final concentration of IgE was calculated using the standard curve.
Ear skins were removed from mice, fixed with 10% formalin, embedded in paraffin wax, routinely processed, and then sectioned into 4 µm thick slices. The skin sections were then stained with hematoxylin & eosin, followed by light microscopy to determine the presence of edema and accumulation of inflammatory cells. Further, thickness levels of the epidermis and dermis were measured using Leica Application Suite (Leica Microsystems, Wetzlar, Germany).
Mast cells were detected by staining with Toluidine blue according to a previously described method [23]. After deparaffinization and dehydration, ear skin sections were stained with 0.25% solution of Toluidine blue (Sigma-Aldrich) and examined by light microscopy for the presence of mast cells. The numbers of cells per specific area in ear tissue sections were measured using Leica Application Suite (Leica Microsystems).
One-way ANOVA was used to verify significant differences between the PA- and No-treated groups (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA). Additionally, response differences between the CMT- or FSC-treated group and vehicle-treated group were evaluated by a post hoc test (SPSS for Windows, Release 10.10, Standard Version, IL, USA) of the variance and significance levels. All values were expressed as the means±SD. A P value of <0.05 was considered significant.
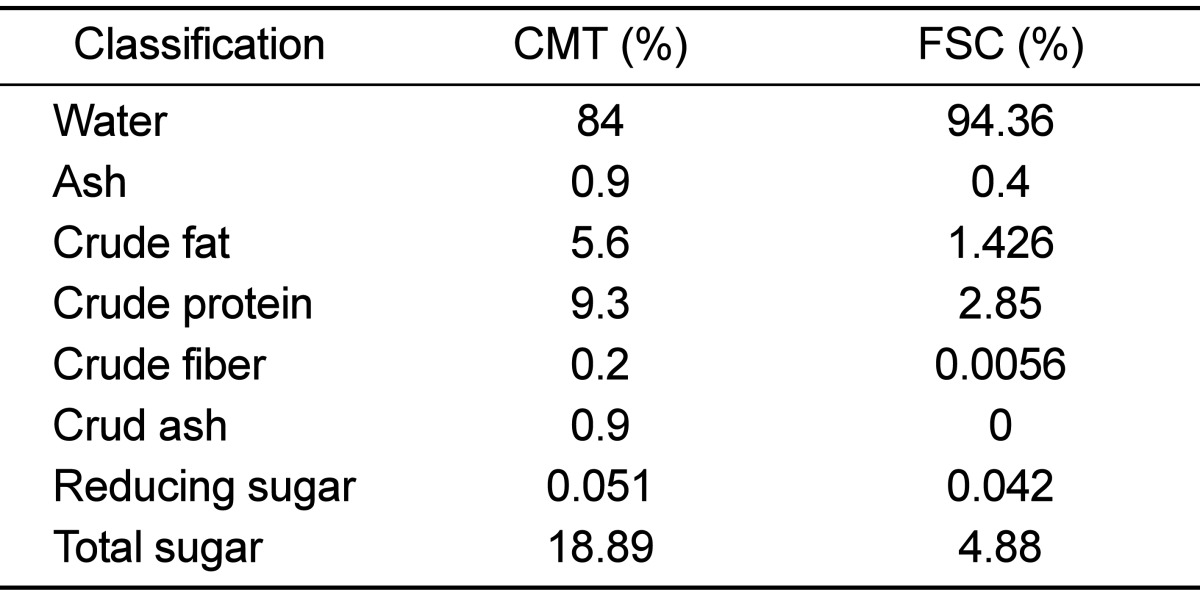
To determine the concentrations of major components in FSC, the levels of several important components, including proteins, fat, fiber, and sugar, were measured using several traditional methods. As shown in Table 1, except for water content, the levels of all components were significantly lower in FSC compared to CMT. Especially, the amounts of crude fiber and ash were reduced by 95-100% CMT, whereas the levels of crude fat and total sugar were reduced by 74-75% CMT. Therefore, the above results indicate that FSC used in this study contained low concentrations of ash, protein, fiber, and sugar.
Firstly, to determine the effects of FSC treatment on the body and organ weights of mice, changes in these factors were measured using an electric balance after sacrifice. No significant difference in body weight was detected in any group after treatment (Figure 1A). Especially, significant alteration of organ weights occurred in the thymus, spleen, and lymph nodes. As shown in Figure 1C and D, PA treatment resulted in elevation of spleen and lymph node weights in ICR mice compared with No treatment. Lymph node weight was significantly reduced in response to either PA+CMT or PA+FSC treatment, although the decrease ratio was greater in PA+FSC-treated mice than in PA+CMT-treated mice. On the other hand, reduction of spleen weight was not detected in either PA+CMT- or PA+FSC-treated mice. The weight of the thymus was lower in PA+vehicle-treated mice than in No-treated mice, whereas it significantly decreased in both PA+CMT- and PA+FSC-treated mice (Figure 1B). These results indicate that FSC may reverse the increase in lymph node weight induced by PA treatment, whereas the other organs did not experience any beneficial changes.
To investigate the effects of FSC on ear phenotypes, changes in ear thickness and morphology were measured in PA+FSC-treated mice. Ear thickness rapidly increased in PA+vehicle-treated mice compared to No-treated mice from days 7 to 14. On the other hand, ear thickness slowly increased in PA+CMT- and PA+FSC-treated mice, although a significant difference could not be detected between the two groups (Figure 2A). Further, the outline of the ear vein became distinct or thickened upon PA+vehicle treatment compared with No treatment, whereas ear color changed from flesh tint to dark brown. However, these changes in ear morphology and ear thickness were slightly reversed upon PA+FSC treatment (Figure 2B). Taken together, our results demonstrate that FSC may successfully induce reduction of ear thickness as well as recovery of ear phenotypes.
Next, we investigated whether or not FSC could suppress elevation of the IgE concentration. To accomplish this, the serum IgE concentration was evaluated in each of the four experimental groups. As shown in Figure 2C, repeated topical application of PA solution resulted in significant elevation of the serum IgE concentration in ICR mice. However, reduction of IgE concentration was observed only in the PA+FSC-treated group, whereas a consistent level was maintained in the PA+CMT-treated group (Figure 2C). Taken together, these results suggest that FSC treatment may contribute to reduction of the IgE concentration in PA-treated ICR mice.
To investigate the suppressive effect of FSC treatment on ear histology, histological analysis of ear tissues from ICR mice was performed. The dermis and epidermis of the ear were thicker in PA+vehicle-treated mice than in No-treated mice. However, epidermal thickness greatly decreased in both the PA+CMT- and PA+FSC-treated groups compared with the PA+vehicle-treated group, although there was no significant difference between them (Figure 3). In addition, significant reduction of dermal thickness was detected only in PA+FSC-treated ICR mice compared with PA+vehicle-treated mice. Especially, PA treatment induced slight changes in the number of adipocytes in the dermal layer of ICR mice. The PA+vehicle-treated group exhibited 4-6-fold higher numbers of adipocytes compared to the No-treated group. On the other hand, FSC treatment resulted in reduction of adipocytes in the dermal region (Figure 3). These results show that FSC may induce reduction of epidermal and dermal thicknesses in ear histology.
Finally, the benefit effect of FSC on infiltration of mast cells was investigated in an ear skin section using toluidine blue staining analysis. Alteration of mast cell numbers was observed under a microscope. Specifically, the number of mast cells stained with blue color was significantly reduced in both the CMT- and FSC-treated groups compared with the No-treated group, whereas numbers were higher in the PA+vehicle-treated group. Especially, the PA+FSC-treated group showed large alteration of the number of master cells (Figure 4). These data indicate that FSC treatment may contribute to the suppression of mast cell infiltration in the dermis of ear skin.
PA is a respiratory sensitizer as well as an important industrial chemical in the large-scale production of plasticizers [24]. However, PA induces inflammation of the eyes in humans due to its occurrence as a vapor, fume, or dust particle. Further, PA treatment sensitizes and irritates the skin and respiratory tract [25,26]. In the case of mice, PA treatment has been shown to increase ear thickness in various types of mice, including BALB/c, C57BL/6, and IL-4/Luc/CNS-1 Tg mice, compared to No treatment [27,28]. In our study, similar results were observed. As shown in Figure 2, ear thickness dramatically increased in ICR mice treated with PA for 2 weeks, and ear phenotypes gradually worsened with time. Meanwhile, PA treatment has previously been shown to increase lymph node weight and IgE concentration in BALB/c and IL-4/Luc/CNS-1 Tg mice [25,27-30]. The results of our study are in agreement with the above reports, although the rates of increase varied. In our study, lymph node weight dramatically increased in the PA+vehicle-treated group, whereas it decreased in the PA+FSC-treated group (Figure 1). Furthermore, PA treatment for 2 weeks induced dramatic elevation of the IgE concentration in ICR mice.
Several studies have shown that intake of fermented soy product or soy sauce containing a high concentration of isolavones can prevent allergic rhinitis [31,32]. Especially, the effect of ImmunoBalance, which is a fermented soy product, was investigated using a NC/TND mouse model of human AD. ImmunoBalance treatment relieved some pathological symptoms of AD, including skin severity score, scratching behavior, cell infiltration, and production of inflammatory cytokines [33]. Furthermore, L. mesenteroides, used in this study to make FSC, may also have the ability to improve allergy symptoms. Specifically, L. mesenteroides was shown to suppress IgE-mediated hypersensitivity in an NC/Nga mouse model [34]. In addition, L. mesenteroides administration to mice has been shown to reduce serum total and ovalbumin (OVA)-specific IgE concentrations while increasing OVA-restimulated IFN-γ secretion in splenocytes [35]. As shown in Figure 2C, FSC containing L. mesenteroides showed similar results in terms of alteration of the IgE concentration. Our results also provide novel evidence that FSC contributes to the alleviation of AD symptoms, although the analytic factors used here differ from those of earlier studies. Particularly, our study focused on alteration of lymph node weight, ear thickness, IgE concentration, and mast cell infiltration, whereas previous studies concentrated on skin morphology, scratching behavioral, and cytokine levels. Further study is needed to investigate the mechanism of action of FSC under various conditions.
Meanwhile, mast cells are considered as a key effector cell type in IgE-mediated immediate hypersensitivity and allergic disorders, as well as in the protection of immune responses to parasites and bacteria [36,37]. However, recent studies have proposed a greater role for mast cells from innate defense against bee and snake venoms [38] to multiple aspects of adaptive immune responses, including antigen presentation, leukocyte recruitment, and draining of lymph nodes [39]. Furthermore, these roles have been extended to allergic diseases, helminth, bacterial infection, autoimmune diseases [40], carcinogenesis [41], allograft tolerance [42], and angiogenesis [43]. Therefore, we investigated alteration of mast cell infiltration in ears of ICR mice in response to PA+FSC treatment. The number of mast cells that infiltrated into the dermis significantly increased in the PA+vehicle-treated group compared to the No-treated group. On the other hand, the number of infiltrated mast cells decreased in the PA+FSC-treated group. This result was very similar to a previous study that investigated the therapeutic effects of Liriope platyphylla in a PA-induced AD mouse model [44,45]. Therfore, mast cells are an important marker to detect the effects of various components on AD therapy.
Taken together, the above results constitute novel findings about FSC function in AD pathology, including ear thickness, IgE concentration and mast cell infiltration. FSC can effectively relieve AD phenotypes induced by PA treatment. Furthermore, these findings indicate that FSC intake can be considered as a new therapeutic strategy for the treatment of AD.
Acknowledgments
We would like to thank Jin Hyang Hwang, animal technician for directing the Animal Facility and Care at the Laboratory Animal Resources Center. This study was supported by a grant from the Korea Institute of Planning Evaluation for Technology of Food, Agriculture, Forestry and Fisheries.
References
1. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.
3. Kay AB. Overview of 'allergy and allergic diseases: with a view to the future'. Br Med Bull. 2000; 56(4):843–864. PMID: 11359624.

4. Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001; 344(1):30–37. PMID: 11136958.
5. Grammatikos AP. The genetic and environmental basis of atopic diseases. Ann Med. 2008; 40(7):482–495. PMID: 18608118.

6. Levy ML. Atopic dermatitis: understanding the disease and its management. Curr Med Res Opin. 2007; 23(12):3091–3103. PMID: 17971284.

7. Simpson EL. Atopic dermatitis: a review of topical treatment options. Curr Med Res Opin. 2010; 26(3):633–640. PMID: 20070141.

8. Pariser D. Topical corticosteroids and topical calcineurin inhibitors in the treatment of atopic dermatitis: focus on percutaneous absorption. Am J Ther. 2009; 16(3):264–273. PMID: 19262357.

9. Draelos ZD. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin. 2008; 24(4):985–994. PMID: 18284804.

10. Cai TD, Chang KC. Dry tofu characteristics affected by soymilk solid content and coagulation time. J Food Qual. 1997; 20:391–402.

11. Cai TD, Chang KC. Characteristics of production-scale tofu as affected by soymilk coagulation method: propeller blade size, mixing time and coagulant concentration. Food Res Intl. 1998; 31(4):289–295.

12. Toda T, Tamura J, Okuhira T. Isoflavone content in commercial soybean food. Foods Food Ingredients J. 1997; 172:83–88.
13. Ashton EL, Dalais FS, Ball MJ. Effect of meat replacement by tofu on CHD risk factors including copper induced LDL oxidation. J Am Coll Nutr. 2000; 19(6):761–767. PMID: 11194529.

14. Oboh G. Coagulants modulate the antioxidant properties and hypocholesterolemic effect of tofu (Curdled soymilk). Asian J Biochem. 2006; 1(1):57–66.

15. Choi YS, Lee SY. Cholesterol-lowering effects of soybean products (Curd or Curd residue) in rats. J Korean Soc Food Nutr. 1993; 22(6):673–677.
16. Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, Okumura K, Goto K, Naito S, Fujimoto K, Hirao Y, Takahashi A, Tsukamoto T, Fujioka T, Akaza H. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004; 95(3):238–242. PMID: 15016323.

17. Wu AH, Ziegler RG, Horn-Ross PL, Nomura AM, West DW, Kolonel LN, Rosenthal JF, Hoover RN, Pike MC. Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev. 1996; 5(11):901–906. PMID: 8922298.
18. Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995; 24(1):33–41. PMID: 7797354.

19. Liu CY, Hsu YH, Wu MT, Pan PC, Ho CK, Su L, Xu X, Li Y, Christiani DC. Kaohsiung Leukemia Research Group. Cured meat, vegetables, and bean-curd foods in relation to childhood acute leukemia risk: a population based case-control study. BMC Cancer. 2009; 9:9–15. PMID: 19134212.

20. Liu J, Chang SK, Wiesenborn D. Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo. J Agric Food Chem. 2005; 53(6):2333–2340. PMID: 15769177.
21. Dubois M, Gillers KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugar and related substance. Anal Chem. 1956; 28:350–352.
22. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959; 31(3):426–428.

23. Kim HJ, Kim J, Kim SJ, Lee SH, Park YS, Park BK, Kim BS, Kim SK, Cho SD, Jung JW, Nam JS, Choi CS, Jung JY. Anti-inflammatory effect of quercetin on picryl chloride-induced contact dermatitis in BALB/c mice. Lab Anim Res. 2010; 26(1):7–13.

24. Hayashi M, Higashi K, Kato H, Kaneko H. Assessment of preferential Th1 or Th2 induction by low-molecular-weight compounds using a reverse transcription-polymerase chain reaction method: comparison of two mouse strains, C57BL/6 and BALB/c. Toxicol Appl Pharmacol. 2001; 177(1):38–45. PMID: 11708898.

25. Ban M, Hettich D. Effect of Th2 cytokine antagonist treatments on chemical-induced allergic response in mice. J Appl Toxicol. 2005; 25(3):239–247. PMID: 15895478.

26. Kimber I, Dearman RJ. The mechanisms and evaluation of chemically induced allergy. Toxicol Lett. 1992; 64-65:79–84. PMID: 1471238.

27. Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR, Lee JW, Lee CK, Hwang DY. IL-6, VEGF, KC, and RNATES are a major cause of a high irritant dermatitis to phthalic anhydride in C57BL/6 inbred mice. Allergol Int. 2010; 59(4):389–397. PMID: 20864798.
28. Bae CJ, Lee JW, Bae HS, Shim SB, Jee SW, Lee SH, Lee CK, Hong JT, Hwang DY. Detection of allergenic compounds using an IL-4/Luciferase/CNS-1 transgenic mice model. Toxicol Sci. 2011; 120(2):349–359. PMID: 21252390.

29. Mori T, Tanimoto Y, Ota M, Masakado T, Kitamoto S, Saito K, Isobe N, Kaneko H. Comparison of cytokine profiles in bronchoalveolar lavage fluid of mice exposed to respiratory and contact sensitizers. J Toxicol Sci. 2012; 37(2):337–343. PMID: 22467024.

30. Fukuyama T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T, Kosaka T. A method for measuring mouse respiratory allergic reaction to low-dose chemical exposure to allergens: an environmental chemical of uncertain allergenicity, a typical contact allergen and a non-sensitizing irritant. Toxicol Lett. 2010; 195(1):35–43. PMID: 20219652.

31. Miyake Y, Sasaki S, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, Hirota Y, Oda H. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: the osaka maternal and child health study. J Allergy Clin Immunol. 2005; 115(6):1176–1183. PMID: 15940131.

32. Kobayashi M. Immunological functions of soy sauce: hypoallergenicity and antiallergic activity of soy sauce. J Biosci Bioeng. 2005; 100(2):144–151. PMID: 16198255.

33. Matsuda A, Tanaka A, Pan W, Okamoto N, Oida K, Kingyo N, Amagai Y, Xia Y, Jang H, Nishikawa S, Kajiwara N, Ahn G, Ohmori K, Matsuda H. Supplementation of the fermented soy product ImmuBalance™ effectively reduces itching behavior of atopic NC/Tnd mice. J Dermatol Sci. 2012; 67(2):130–139. PMID: 22748506.

34. Masuda Y, Takahashi T, Yoshida K, Nishitani Y, Mizuno M, Mizoguchi H. Anti-allergic effect of lactic acid bacteria isolated from seed mash used for brewing sake is not dependent on the total IgE levels. J Biosci Bioeng. 2012; 114(3):292–296. PMID: 22652086.

35. Kang H, Myung EJ, Ahn KS, Eom HJ, Han NS, Kim YB, Kim YJ, Sohn NW. Induction of Th1 cytokines by Leuconostoc mesenteroides subsp. mesenteroides (KCTC 3100) under Th2-type conditions and the requirement of NF-kappaB and p38/JNK. Cytokine. 2009; 46(2):283–289. PMID: 19299163.
36. Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009; 21(6):666–678. PMID: 19828304.

38. Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006; 313(5786):526–530. PMID: 16873664.

39. McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003; 4(12):1199–1205. PMID: 14595438.

40. Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000; 191(5):813–822. PMID: 10704463.

41. Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007; 13(10):1211–1218. PMID: 17906636.

42. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Snick JV, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006; 442(7106):997–1002. PMID: 16921386.

43. Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005; 202(6):739–750. PMID: 16157686.

44. Kim JE, Hwang IS, Goo JS, Nam SH, Choi SI, Lee HR, Lee YJ, Kim YH, Park SJ, Kim NS, Choi YH, Hwang DY. LP9M80-H isolated from Liriope platyphylla could help alleviate diabetic symptoms via the regulation of glucose and lipid concentration. J Life Sci. 2012; 22(5):634–641.
45. Kim JE, Lee YK, Nam SH, Choi SI, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. The symptoms of atopic dermatitis in NC/Nga mice were significantly relieved by the water extract of Liriope platyphylla. Lab Anim Res. 2010; 26(4):377–384.
Figure 1
Differences in body (A) and organ (B-D) weights of ICR mice. Body weights of mice in each of the four groups were measured using a chemical balance over 2 weeks. After final application, the weights of three organs, including the thymus, spleen, and lymph nodes, were measured by following the procedure described in Materials and Methods. Data shown are the means±SD (n=5). a, P<0.05 is the significance level compared to the NO-treated group. b, P<0.05 is the significance level compared to the PA+vehicle-treated group.
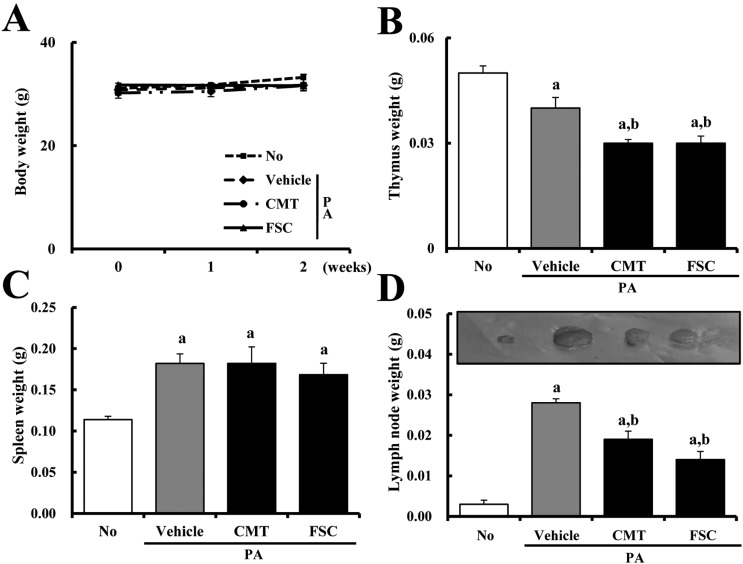
Figure 2
Differences in ear thickness (A), ear phenotypes (B), and IgE concentration (C). PA solution was repeatedly applied to the dorsum of ears of ICR mice during oral gavage of FSC. After 2 weeks, ear thickness and phenotypes were observed by following the procedure described in Materials and Methods. Serum used to measure the IgE concentration was prepared from blood samples collected from the abdominal veins of mice. Serum IgE concentration was quantified using an IgE ELISA kit, which has an IgE sensitivity of 1 ng/mL and a detection range from 1 ng/mL to 100 ng/mL. Data shown are the means±SD (n=5). a, P<0.05 is the significance level compared to the No-treated group. b, P<0.05 is the significance level compared to the PA+vehicle-treated group.

Figure 3
Histopathology of ear skin of ICR mice treated with No, PA+vehicle, PA+CMT, or PA+FSC. PA solution was repeatedly applied to the dorsum of ears of ICR mice during oral gavage of FSC. After 2 weeks, histological changes were observed as described in Materials and Methods. (A) Slide sections of ear tissue were stained with hematoxylin & eosin and observed at a magnification of 200x. (B) Epidermal and dermal thicknesses in the histological section were measured using Leica Application Suite. Data shown are the means±SD (n=5). a, P<0.05 is the significance level compared to the No-treated group. b, P<0.05 is the significance level compared to the PA+vehicle-treated group.
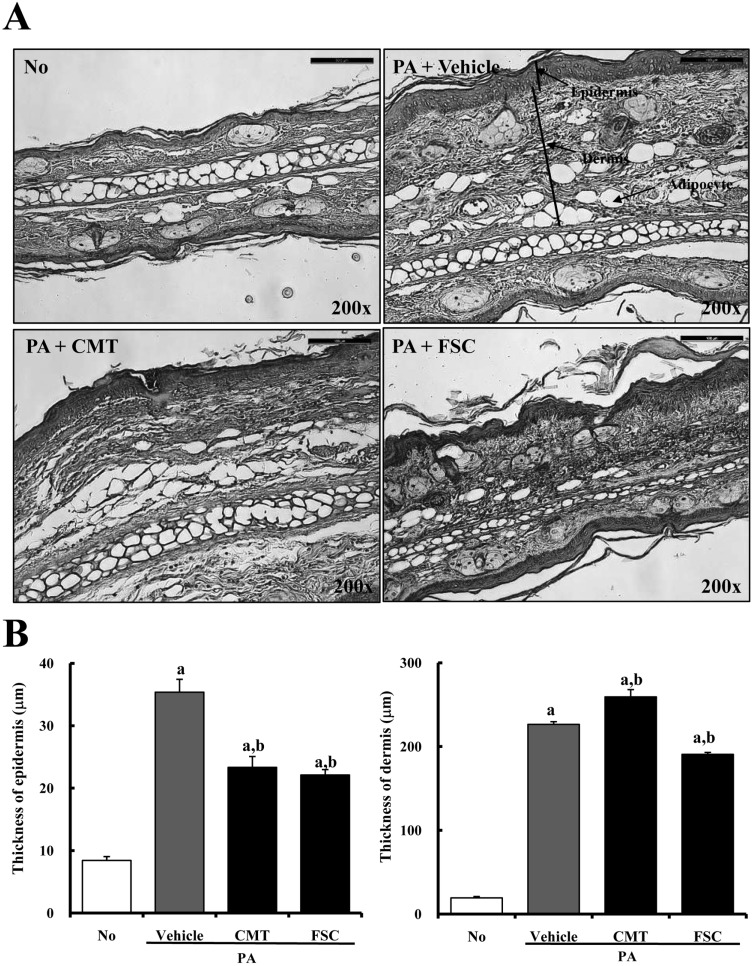
Figure 4
Infiltration of mast cells of ICR mice treated with No, PA+vehicle, PA+CMT, or PA+FSC. (A) Slide sections of ear tissue were stained with 0.25% toluidine blue and observed at a magnification of 400x. Arrowhead indicates infiltrated mast cells in the dermis of ears. (B) Numbers of infiltrated mast cells in the stained sections were detected using Leica Application Suite. Data shown are the means±SD (n=5). a, P<0.05 is the significance level compared to the No-treated group. b, P<0.05 is the significance level compared to the PA+vehicle-treated group.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download