Abstract
Diabetes mellitus is a major predictor of heart failure, although the mechanisms by which the disease causes cardiomyopathy are not well understood. The purpose of this study was to determine whether prolonged exposure of cardiomyocytes to high glucose concentrations induces autophagy and contributes to cardiomyopathy. Interestingly, there were no differences in the autophagic activation produced by different glucose concentrations. However, cell viability was decreased by high glucose. In the diabetic rats, we found a higher level of microtubule-associated protein light chain 3 (LC3) expression and a reduction in the size of the left ventricle (LV) (P<0.05) caused by growth retardation, suggesting activated autophagy. Our in vitro findings indicate that hyperglycemic oxidative stress induces autophagy, and our in vivo studies reveal that autophagy is involved in the progression of pathophysiological remodeling of the heart. Taken together, the studies suggest that autophagy may play a role in the pathogenesis of juvenile diabetic cardiomyopathy.
Diabetic cardiomyopathy has become a major cause of disability and mortality in diabetic patients [1,2]. Diabetic cardiomyopathy is the presence of myocardial dysfunction in the absence of coronary artery disease and hypertension [3], suggesting that diabetes has a direct effect on cardiomyocytes. Several studies have shown that hyperglycemia is an independent risk factor that directly causes cardiac damage, leading to diabetic cardiomyopathy [4-7]. However, the mechanisms by which hyperglycemia causes cardiac damage remain unclear.
Hyperglycemia appears to trigger a series of maladaptive stimuli that result in myocardial fibrosis and collagen deposition [3,8]. These chronic changes are believed to result from the initial response of the myocardium to a sudden increase in glucose levels [4,9], which can manifest as metabolic abnormalities, subcellular defects, the abnormal expression of genes [9-11], and, ultimately, lead to cardiac cell death [9,12,13]. Recent studies showed a higher rate of apoptosis in the hearts of patients with diabetes [14] and in animals with streptozotocin (STZ)-induced diabetes [15]. In an in vitro study, cardiomyocytes exposed to high levels of glucose also showed increased apoptosis rates [13,16]. However, it is not clear whether the increased myocardial autophagic cell death observed in these studies was related directly to hyperglycemia.
Chronic hyperglycemia can cause oxidative stress [9] and endoplasmic reticulum (ER) stress [17,18]. ER stress is particularly prevalent in diabetes, causing an overall increase in protein misfolding [19]. Recently, oxidative stress has been demonstrated to activate autophagy in rat hepatocytes and mouse fibroblasts [20]. Autophagy is an intracellular bulk degradation process in which long-lived proteins and organelles in the cytosol are degraded and recycled [21]. The activation of autophagy at the early stages of diabetes may be a protective mechanism for scavenging and eliminating misfolded, polyubiquitinated protein aggregates [22]. These findings suggest that autophagy is stimulated in response to diabetic conditions, although it has not been shown to occur in diabetic cardiomyocytes.
Autophagy occurs at basal levels but can be further induced by stresses such as nutrient depletion [23]. Autophagy also promotes programmed cell death in some circumstances [24,25], suggesting that it plays a dual role in cell survival. In diabetes, however, it is not known whether autophagy is protective and necessary for the survival of cardiac and skeletal myocytes, or if it instead mediates cell death during pathologically relevant stresses.
Increased autophagosomes have been found in an in vivo analysis of autophagy in response to nutrient starvation, as a mimic state of infarction, in transgenic mice expressing a fluorescent autophagosome marker, microtubule-associated protein-1 light chain-3 (LC3) [26]. Two forms of LC3, called LC3-I and LC3-II, were produced post-translationally in various cells. LC3-I is cytosolic, whereas LC3-II is a processed form located on the autophagosomal membrane; it is the first mammalian protein to be identified that specifically associates with this membrane. The LC3-II/LC3-I ratio is correlated with the extent of autophagosome formation [27].
The number of autophagy-related publications in the cardiovascular research has increased considerably during the past 2 years, indicating that autophagy is becoming a topic of major importance. However, compared to apoptosis and the ubiquitin-proteasome proteolytic pathway, autophagy remains an underestimated and largely neglected phenomenon in the research on cardiac and skeletal muscular atrophy. The present study investigated whether a high glucose state could induce autophagy in the rat cardiomyocyte cell line H9c2 and examined the role of autophagy in diabetes-induced cardiac myopathy in juvenile rats.
Cardiac H9c2 cells (undifferentiated neonatal rat cardiomyocytes) were obtained from the Korean Cell Line Bank (Seoul, Korea; KCLB No. 21446) and cultured as previously described [21,23,24,28]. H9c2 cells from passages 18-20 in the actively growing condition were used for experiments. In some experiments, the cells were grown in chamber slides, which were used for immunostaining, MDC staining, and PI staining.
To assess cell division, cells were seeded at a density of 2×104/mL in a 24-well dish. After 12 hr, the cultures were exposed to a final concentration of 4.5 g/L D-glucose (16.5mM) as control; 9 g/L D-glucose, representing a two-fold higher glucose concentration (33 mM glucose medium); and 18 g/L, representing a four-fold higher glucose level (66 mM glucose medium). DMEM with no glucose (GIBCO Invitrogen 11966, Grand Island, NY, USA) was used for the glucose-free treatment. After exposure for varying time periods, the monolayer cultures were washed with PBS, and the cells were removed by trypsinization. Then, viable cells were harvested and counted at 24 hr intervals for 7 days using a trypan blue assay and a hemocytometer.
To assess cell viability, cells were seeded at a density of 2×104/mL in a 60 mm culture dishes. When cell populations reached 40~50% confluence, the cultures were exposed to D-glucose in final concentrations of 9 g/L and 18 g/L for in vitro treatment with two-fold and four-fold higher glucose levels, respectively, and exposed to 4.5 g/L D-glucose as control, according to previously described [29-31]. After trypsinization of the cells, then, viable cells were harvested and counted at 24 hr intervals for 3 days using a trypan blue assay and a hemocytometer.
The specific-pathogen-free 4-week-old male Sprague-Dawley (SD) rats were purchased from Samtaco BioKorea (Osan, Korea). They were housed two per cage under controlled environmental conditions (23℃, 55% humidity) with an established photoperiod of 12 hr light/day (lights on 06:00 hr) in an accredited facility, Animal Resource Center of Inje University, which has been monitored regularly for microbiology infection. They had free access to standard rodent chow and tap water ad libitum. all in-vivo studies were reviewed and approved by the Ethics Committee for Animal Care and Use of Inje University, which is certified by the Korean Association for Accreditation of Laboratory Animal Care. Animals were divided into two groups; a control group (n=5), a STZ-treated diabetic group (n=7). They were maintained for the following 4 weeks before sacrifice.
Rats were given a single intraperitoneal (IP) dose of STZ, a well-known specific pancreatic β-cell toxin, dissolved in a sodium citrate buffer (pH 4.5) at an initial dose of 60 mg/kg body weight. After 3 days, blood glucose levels were determined with a glucometer (Super Glucocard II, ArkRay, Kyoto, Japan). Animals with blood glucose levels below 200 mg/dL were supplemented with additional STZ via intraperitoneal injection and reassessed. Rats with blood glucose levels higher than 200 mg/dL were considered to have moderate diabetes. Animals that were unresponsive to treatment (blood glucose <200 mg/dL) were omitted from the study. Blood samples were obtained by venipuncture of a tail vein with a glucometer.
All animals were weighed and anesthetized with a mixture of ketamine (50 mg/kg body weight) and xylazine (10 mg/kg body weight). In each rat, the heart and tibia were collected and stored frozen at -70℃ until analysis. Upon analysis, the heart was carefully weighed to the nearest 0.01 g on an electronic balance. The left ventricle (LV), including the interventricular septum, was isolated by removing the atria and the free wall of the right ventricle, and weighed. Both the right and left tibia were removed and cleaned of muscle and connective tissue. Tibial length (TL) was measured from the top of the tibial plateau to the bottom of the lateral malleolus process with vernier calipers (Mitutoyo, Japan).
Routine histologic analysis was performed using cryostat sections at 10 µm thickness. Hematoxylin-eosin preparations of each specimen were examined with an Olympus BX51 microscope. Diameters of cardiac muscle fibers were measured from cross-sectioned images captured with scale using DP-70 camera and DP-Controller software, and further processed using DP-Manager (Olympus, Tokyo, Japan).
For immunofluorescence analysis, the sections were incubated at room temperature for 2 hr with a polyclonal goat antibody against LC3 (diluted 1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA), with FITC red-labeled horse anti-goat IgG (diluted 1:100; Santa Cruz Biotechnology) for 1 hr, then rinsed and mounted on slides for visualization by fluorescence microscopy. Images were captured using Olympus BX51 microscope and DP71 digital camera and software (Olympus, Tokyo, Japan).
Data were collected from three repeated experiments, and presented as mean±S.E.M. One-way analysis of variance (ANOVA) and Student's t-test were used for statistical analyses. Differences were considered to be significant P<0.05. All analyses were performed using the Statistical Package for the SPSS ver.18 software (SPSS Inc., Chicago, IL, USA).
We searched for the induction of autophagy in H9c2 cells, indicating starvation conditions, in vitro. Figure 1A shows representative inverted microscopy images taken after 12 hr of glucose starvation. In glucose-starved cells, we observed a significant reduction in overall cell size and in the size of the cytoplasm, suggesting a cellular response to the stress. In glucose-deprived cells, LC3-II/LC3-I was significantly elevated compared to control cells in complete media (Figure 1B).
Next, we examined the effect of high glucose to viability of the cardiomyocytes, and autophagy. The incubation of H9c2 cardiomyocytes in glucose-free medium for 72 hr caused cell death about 30% (Figure 2A, B). Incubation under four-fold higher glucose levels, also accelerated cell death at the similar level. To further evaluate the effects of the glucose environment on the formation of autophagosomes, we examined the formation of LC3-II in different glucose concentration, and different time points of high glucose condition by immunoblot assay (Figure 2C). As we expected, glucose deprivation induced significantly increased autophagy. However, four-fold higher glucose conditions also markedly increased LC3-II/LC3-I ratio after 72 hr, compared to control (**P<0.01).
We induced diabetes in 4-week-old rats to investigate the effect of diabetes on heart development; the rats maintained a diabetic state for 4 weeks. Figure 3 shows a comparison of the general characteristics of the control and diabetic rats. The body weight of the diabetic rats was significantly lower (*P<0.001) than that of the control rats (Figure 3A). However, blood glucose levels were higher in diabetic rats compared to control rats (*P<0.01; Figure 3C). The systolic tail artery blood pressure of conscious rats was not significantly different between diabetic and control rats.
Comparisons of physiologic and morphometric findings in control and diabetic rats are shown in table 1. The LV mass normalized by TL was significantly smaller in diabetic rats (13.815±0.931) than in control rats (18.736±4.565; *P<0.05). Morphological and morphometric findings are shown in Figure 4. Following 4 weeks of observation, both LV/body weight (BW) and LV/TL were considerably smaller in diabetic rats than in control rats, as was the thickness of the LV wall (Figure 4, Table 1).
Histological examination of hematoxylin-eosin stained sections from the cardiac muscles of normal and diabetic rats showed a marked reduction of myofiber thickness (Figure 4C,D). Staining of nuclei with propidium iodide (PI) showed an increase in the number of nuclei with many orange dots in STZ-treated group compared to control, which represents more dense distribution of myonuclei due to reduced size of myocytes. Occasionally, small areas of fibrosis, denoted by white spaces, were seen among fibers.
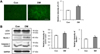
A number of punctuates, which represent autophagosomes stained with LC3, were detected by immunohistochemistry (Figure 5A). Immunoblot analysis of heart tissues shows that the expression of beclin 1, an upstream molecule for autophagy, was induced in diabetic rats, as well as elevated level of LC3-II (Figure 5B). In addition, cleaved form of caspase 3, one of a family of proteins that are sequentially activated to produce apoptosis, was slightly augmented in diabetic animals, although it was not statistically significant.
The present study confirmed that, compared to control rats, diabetic rats had lower body weight (caused by growth retardation), LV size, and tibia length, but higher blood glucose levels. According to a previous study, diabetes causes slower digestion of nutrients and slower growth during the pubertal period because of abnormalities in the growth hormone (GH) and insulin-like growth factor (IGF-1) axis that lead to impairment to hepatic growth hormone receptors [32]. Moreover, intensive insulin treatment have led to a reduction in diabetic complications and ameliorate growth in children with type I diabetes via GH - IGF-1 axis [33]. In the present study, we found notable growth retardation in the heart of diabetic rats (Figure 4, Table 1). Compared to control rats, the LV/BW and LV/TL values were lower in diabetes, suggesting severe impairment to cardiac growth, which might progress to diabetic cardiomyopathy. Although our data cannot verify the role of autophagy in diabetic cardiomyopathy, we did find increased expression of LC3, as shown in Figure 3. These results suggest the stimulation of autophagy in the diabetic myocardium, although the specific factors that trigger autophagy are not known.
In an in vitro study, we investigated hyperglycemic stress using H9c2 cardiomyoblasts. Hyperglycemia is known to induce autophagy and has been shown to directly causes cardiac damage, leading to diabetic cardiomyopathy [4-7]. Indeed, cell death, which is a consequence of the abnormal cellular metabolism and gene expression that results from hyperglycemia, has been considered an important cause of cardiomyopathy [11,13,34,35]. Our result showed incubation under four-fold higher glucose levels (66 mM), also accelerated cell death as well as glucose deprivation (Figure 2A-B). 66 mM of glucose in vitro experimental condition represents hyperglycemia [16], suggesting that the death of H9c2 cells occurs during both glucose deprivation and in a high glucose environment. Moreover, we proposed that autophagy is associated with long-term exposure to high glucose environment (Figure 2C-D). These results implicate that cardiomyocytes might be detrimental in high glucose environment, and autophagy might be involved to mediate cell death or protect cellular homeostasis.
The results of our in vitro study parallel the results of our study of diabetic animals. In the diabetic state, insulin dysfunction or insulin deficiency causes the impairment of glucose uptake through the cell membrane, and, although blood glucose levels are high, cardiomyocytes (and other cells) suffer glucose starvation. In this study, we used different concentrations of glucose media to investigate whether glucose deficiency and high extracellular glucose conditions influence autophagy. As observed in previous studies, autophagy was strongly induced in cells subjected to glucose deprivation (Figure 2). Interestingly, high glucose media also induced autophagy, accompanied by cell death. We do not know if cell death was the result of apoptosis or autophagy. Comparative studies of apoptosis and autophagy in different glucose concentration are necessary to determine if autophagy is adaptive or maladaptive in these situations.
High glucose levels are known to increase superoxide anion generation and to trigger apoptosis [13], and oxidative stress induces autophagy [20] respectively. It is possible that high glucose-induced reactive oxidative species generation is associated with the induction of autophagy in cardiomyocytes. Our results showed that diabetes induced increased expression of Beclin-1 and LC3-II (Figure 5B), which are essential molecules for autophagy. This suggests that autophagy contributes to diabetic cardiomyopathy. Several studies pointed out apoptosis in the hearts of patients with diabetes [14] and in STZ-induced diabetic rats [36]. We tried to examine the activation of caspase 3 as an apoptosis marker. Western blot assay shows slightly augmented, but not significantly different (Figure 5B). Because other studies demonstrated 2.5-fold higher caspase-3 activity, using aminomethylcoumarin (AMC)-derived substrate Z-DEVD-AMC [36,37] and 5-fold increase in apoptosis, using TUNNEL assay, in the diabetic heart [38]. In this study, It might be a limitation to show evident difference of cleaved caspase-3 in cardiac tissue between diabetic and control groups by Western blot assay only, but other assays for evaluating apoptosis tends to be consistent with increased caspase-3 activity of cardiac muscle in STZ-induced diabetic animal model. Taken together, increased apoptosis of cardiomyocytes in diabetic condition might be also associated with activation of autophagy. Further investigation is necessary to elucidate the molecular mechanisms of diabetes-associated autophagy in diabetic cardiomyocytes, supposed to be apoptotic cell death.
This study is the first to demonstrate that autophagy, a process that causes intracellular protein degradation, is induced in a high glucose environment and by long-term glucose exposure, and it is activated in the myocardium of type I diabetic rats. More importantly, we found that STZ-induced diabetes upregulates LC3, a component of the autophagosomal membrane, in cardiac muscle tissue. Since it is still unclear whether autophagy is an adaptive or maladaptive mechanism in the diabetic state, further studies that control autophagy, for example, using transgenic animals or the overexpression of autophagy-related genes that then induce diabetes, are needed.
Figures and Tables
Figure 1
Glucose starvation strongly induces cell-size reduction and autophagy. A, Representative inverted microscopy images after 12 h of glucose starvation. Magnification ×100. Scale bar=40 µm. B, Immunoblot analysis of microtubule-associated protein-1 light chain-3 (LC3)-I and LC3-II. Cardiac myocytes were incubated with complete media (control) or treated with glucose-free [Glu(-)] medium for 12 h. In A and B, the results represent data from three independent experiments. **P<0.01
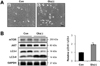
Figure 2
High glucose media suppresses proliferation of cardiomyocytes, and induces cell death with accompanied by autophagy. A, Representative light microscopy images of H9c2 cells. Cells were treated with complete media as a control (Con) or with glucose-free [Glu(-)], 33 mM glucose [Glu(2×)], or 66 mM glucose [Glu(4×)] media for 36 hr and 72 hr. Scale bar = 100 um. B, Time course of cell viability after media exchange, estimated by trypan blue assay. Values are mean from three independent experiments. **P<0.01 vs. Con. C, Immunoblot analysis of LC3-I and LC3-II. Cells were cultured in complete media as a control (Con), glucose-free media [Glu(-)], 33 mM glucose [Glu(2×)], or 66 mM glucose [Glu(4×)] for 72 hr, and quantitative analysis of relative LC3-II expression to compare the level of autophagy between groups. *P<0.05 vs. Con, **P<0.01 vs. Con. D, Time course of LC3 expression by immunoblot analysis after 66 mM glucose [Glu(4×)] treatment, and quantitative analysis of relative LC3-II expression to compare the level of autophagy between time points in Glu(4×) group. *P<0.05 vs. Con
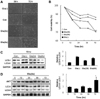
Figure 3
General features of diabetic rats. A, Body weight of STZ-induced diabetic vs. control rats. *P<0.01 B, Blood pressure of STZ-induced diabetic vs. control rats. C, Blood glucose level of STZ-induced diabetic vs. control rats. *P<0.01 D, Measurement of tibia length in control and diabetic rats after 14 and 28 days of STZ treatment. In comparison to the untreated control, the diabetic rats show suppressed bone growth. *P<0.05.

Figure 4
The size of the left ventricle is reduced in diabetic rats with cardiac muscle atropy. After 4 weeks, three control and three diabetic animals were sacrificed. A, Representative morphological images of wet left ventricular tissue showing that early-onset diabetes causes the suppression of heart development. (a, b) Representative cross sections of the heart showing the reduced thickness of the left ventricular wall. Scale bar=0.5 cm. (c, d) B, The effects of diabetes on the left ventricle mass to body weight (LV/BW) index (upper panel), and the ratio of the left ventricle mass to tibia length was also decreased (lower panel). *P<0.05 C, Hematoxylin-eosin (H&E) stained cross-sections of rat heart muscles after 28 days of streptozotocin treatment (DM) or vehicle treatment (Con). Distribution of nuclei was further confirmed by propidium iodide (PI) staining. Magnification ×400, scale bar=50 µm. D, Comparative analysis of the diameter of muscle fibers between groups. *P<0.05 vs. Con
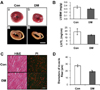
Figure 5
Diabetes-induced myocardial autophagy. A, Immunohistochemistry using antibody to microtubule-associated protein-1 light chain-3 (LC3). Increased abundance of the autophagosomal marker, LC3, in diabetic rats compared to control rats. Magnification ×100, scale bar=100 µm. B, Representative immunoblots probed for LC3, beclin 1, and caspase 3 in ventricular lysates, as indicated. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Acknowledgment
This research was supported by grant from KRIBB Research Initiative Program (KGM0321112), Republic of Korea.
References
1. Trost S, LeWinter M. Diabetic Cardiomyopathy. Curr Treat Options Cardiovasc Med. 2001. 3(6):481–492.
2. Giles TD. The patient with diabetes mellitus and heart failure: atrisk issues. Am J Med. 2003. 115(Suppl 8A):107S–110S.
3. Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008. 121(9):748–757.
4. Sowers JR. Johnstone MT, Veves A, editors. Diabetes and hypertension. Contemporary cardiology: Diabetes and cardiovascular disease. 2001. 1st ed. Totowa, N.J.: Humana Press;123–129.
5. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972. 30(6):595–602.
6. Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000. 101(19):2271–2276.
7. Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol. 2000. 86(3):309–312.
8. Kawasaki D, Kosugi K, Waki H, Yamamoto K, Tsujino T, Masuyama T. Role of activated renin-angiotensin system in myocardial fibrosis and left ventricular diastolic dysfunction in diabetic patients--reversal by chronic angiotensin II type 1A receptor blockade. Circ J. 2007. 71(4):524–529.
9. Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001. 1(3):181–193.
10. Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, Davies PJ, Taegtmeyer H. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000. 32(6):985–996.
11. Pawelczyk T, Sakowicz M, Szczepanska-Konkel M, Angielski S. Decreased expression of adenosine kinase in streptozotocin-induced diabetes mellitus rats. Arch Biochem Biophys. 2000. 375(1):1–6.
12. Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999. 79(1):215–262.
13. Feuerstein GZ, Young PR. Apoptosis in cardiac diseases: stress- and mitogen-activated signaling pathways. Cardiovasc Res. 2000. 45(3):560–569.
14. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000. 87(12):1123–1132.
15. Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001. 50(6):1414–1424.
16. Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002. 51(6):1938–1948.
17. Unger RH. Lipotoxic diseases. Annu Rev Med. 2002. 53:319–336.
18. Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005. 118(Pt 17):3905–3915.
19. Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005. 280(1):847–851.
20. Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004. 15(11):4829–4840.
21. Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004. 6(4):463–477.
22. Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007. 117(7):1782–1793.
23. Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005. 6(6):439–448.
24. Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004. 304(5676):1500–1502.
25. Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004. 6(12):1221–1228.
26. Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004. 15(3):1101–1111.
27. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000. 19(21):5720–5728.
28. Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX, Lin SG, Li Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008. 376(3):548–552.
29. Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhäusl W. High-glucose--triggered apoptosis in cultured endothelial cells. Diabetes. 1995. 44(11):1323–1327.
30. Du XL, Sui GZ, Stockklauser-Färber K, Weiss J, Zink S, Schwippert B, Wu QX, Tschöpe D, Rösen P. Introduction of apoptosis by high proinsulin and glucose in cultured human umbilical vein endothelial cells is mediated by reactive oxygen species. Diabetologia. 1998. 41(3):249–256.
31. Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation. 2000. 101(22):2618–2624.
32. Bornfeldt KE, Arnqvist HJ, Enberg B, Mathews LS, Norstedt G. Regulation of insulin-like growth factor-I and growth hormone receptor gene expression by diabetes and nutritional state in rat tissues. J Endocrinol. 1989. 122(3):651–656.
33. Chiarelli F, Giannini C, Mohn A. Growth, growth factors and diabetes. Eur J Endocrinol. 2004. 151:Suppl 3. U109–U117.
34. Teiger E, Than VD, Richard L, Wisnewsky C, Tea BS, Gaboury L, Tremblay J, Schwartz K, Hamet P. Apoptosis in pressure overload-induced heart hypertrophy in the rat. J Clin Invest. 1996. 97(12):2891–2897.
35. Kang YJ. Molecular and cellular mechanisms of cardiotoxicity. Environ Health Perspect. 2001. 109:Suppl 1. 27–34.
36. Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II-dependent. Lab Invest. 2000. 80(4):513–527.
37. Ghosh S, Qi D, An D, Pulinilkunnil T, Abrahani A, Kuo KH, Wambolt RB, Allard M, Innis SM, Rodrigues B. Brief episode of STZ-induced hyperglycemia produces cardiac abnormalities in rats fed a diet rich in n-6 PUFA. Am J Physiol Heart Circ Physiol. 2004. 287(6):H2518–H2527.
38. Ghosh S, An D, Pulinilkunnil T, Qi D, Lau HC, Abrahani A, Innis SM, Rodrigues B. Role of dietary fatty acids and acute hyperglycemia in modulating cardiac cell death. Nutrition. 2004. 20(10):916–923.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download