Abstract
The anti-inflammatory effects of fuciodan and Cistanche tubulosa (CT) extract were investigated in vitro macrophage culture system and in vivo carrageenan-induced air pouch inflammation model. CT extract inhibited nitric oxide production from activated RAW 264.7 macrophage cells, while fucoidan was inactive. In vivo air pouch inflammation model, carrageenan-induced vascular exudation and increased nitric oxide and prostaglandin E2 concentrations in the exudates were synergistically suppressed by co-administration of fucoidan or CT extract. Moreover, tissue inflammation was substantially attenuated by the combinational therapy. However, there was no synergistic effect against the inflammatory cell infiltration, although fucoidan and CT extract each markedly reduced the cell numbers. Therefore, it is suggested that fucoidan blocks infiltration of inflammatory cells, while CT extract inhibits activation of the cells, and that their combinational treatment could be a promising candidate for the relief of various types of inflammation.
As an immune response against foreign antigens, macrophages release pro-inflammatory cytokines such as tumor-necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and others [1,2]. Such cytokines induces chemotactic influx of granulocytes, monocytes, lymphocytes, and mast cells to damaged tissue that promotes antigen removal and tissue recovery [3,4]. However, excessive infiltration and activation of the cells aggravates tissue injuries, leading to edema (vascular exudation) and pain which are well-known phenomena of inflammation.
TNF-α is an important factor for inducing nitric oxide synthase (iNOS) gene expression in several cell lines. iNOS activation leads to nitric oxide (NO) production [5-7] that not only modulates several physiological functions such as bactericidal and vasodilatation, but also causes inflammation [8,9]. After tissue injury, degradation by phospholipases of cell membrane phospholipids generates arachidonic acid. Arachidonic acid is further decomposed by cyclooxygenase (COX) to prostaglandins (PGs) [10]. Excessive amount of PGE2 formed by COX-II induces several cytokines for inflammation. Since both NO and PGE2 act as major factors for inflammation and pain induction, TNF-α-NO and COX-II-PGE2 pathways are main streams of inflammatory process, which are inhibited by corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs), respectively [10,11].
Even though there are several steroids and NSAIDs for the therapy of inflammatory diseases, these drugs may give rise to adverse effects on the immune system, gastrointestinal tract, kidneys, liver, central nervous system, blood pressure and cardiovascular system [12-14]. Therefore, it is necessary to minimize the adverse effects of chemical therapeutics by replacing them or using in combination with natural products.
Fucoidan, sulfate polysaccharide complex from seaweeds such as Laminaria japonica and Cladosiphon okamuranus widely distributed in many countries, has been used as therapeutics in Oriental medicine for long period. In previous studies, it has been demonstrated that fucoidan has anti-oxidative, anti-coagulative, anti-cancer, and anti-inflammatory activities [15,16]. Accordingly, the beneficial effects of fucoidan on inflammatory diseases, ischemia, immune dysfunction and tumors are attracting investigators' attention [17,18]. On the other hand, Cistanche tubulosa (CT) has also been widely used for traditional medication in China. It was reported that CT extract lower the production of TNF-á and IL-4 that are major factors for NO production in inflammatory pathway [19].
Such previous findings led us to investigate the combinational efficacy of fucoidan and CT extract on the 2 major pathways of inflammation. In order to elucidate the mechanism of action, we analyzed NO and PGE2 in the exudates from carrageenan-induced air pouch inflammation [20].
Semi-purified fucoidan and water extract of CT were obtained from Misuba RTech Co., Ltd. (Asan, Korea). Fucoidan and CT extract were kept at 4℃, mixed (1:3) and dissolved in purified water before use, and orally administered in a volume of 5 mL/kg.
The murine macrophage RAW 264.7 cell line was purchased from the American Type Culture Collection (Manassas, USA), and cultured in Dulbecco's modified Eagle's medium (Sigma, St. Louis, USA) containing 10% fetal bovine serum and antibiotics [100 U/mL penicillin (Sigma) and 100 µg/mL streptomycin (Sigma)]. The cells were incubated in a humidified 5% CO2 atmosphere at 37℃.
To assess the effects of fucoidan and CT extract on the secretion of NO, RAW 264.7 cells (1×106 cells/mL) were incubated with fucoidan or CT extract (1-320 µg/mL) for 5 min, followed by interferon-γ (IFN-γ, 10 U/mL) and lipopolysaccharide (LPS, 10 µg/mL) for 24 hours. In our preliminary studies, it was confirmed that treatment with IFN-γ plus LPS for the activation of RAW 264.7 cells did not affect viability of the cells up to 24 hours, and that fucoidan and CT extract were not cytotoxic up to 1 mg/mL. The concentration of nitrite (NO2-), the oxidized product of NO, was measured as an indicator of NO. Culture medium was mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamide in 2.5% phosphoric acid) [21], and incubated at room temperature for 10 min. Nitrite concentration was analyzed at 540 nm, where NaNO2 was used to generate a standard curve.
Male ICR mice (7 weeks old) were purchased from the Daehan Biolink (Eumseong, Korea), and housed in a room with constant environmental conditions (22±2℃; 40-70% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). Pellet feed and purified water were available ad libitum. All the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University (CBNU), Korea, and conducted according to the Standard Operation Procedures (SOP) of the Laboratory Animal Research Center, CBNU.
The mice (n=8/group) were orally treated with fucoidan (18 or 54 mg/kg), CT extract (54 or 162 mg/kg), or their mixture (18+54, 54+162 or 90+270 mg/kg) once a day for 7 days. Low doses of fucoidan (18 mg/kg) and CT extract (54 mg/kg) were from estimated human doses of 100 mg/body (70 kg) and 300 mg/body after surface area translation of body weight (Km=37 for human/Km=3 for mouse), respectively. High doses of fucoidan and CT extract were set at 3 folds, alone or in mixture, and the highest-dose mixture was fixed at 5 folds to assess maximum synergistic effects.
On the 1st day of fucoidan and CT extract treatment, mice were subcutaneously injected with 10 mL of sterile air into the back side to form a pouch [22,23]. After 2 and 5 days, the pouch was re-injected with 5 mL of air. One hour after the final treatment, 1 mL of carrageenan (1% in saline; Sigma) or its vehicle (saline) was injected into the pouch.
The air pouch was washed with 1 mL of cold saline 6 hours after carrageenan injection, and the net volume of lavage fluid was recorded. Total numbers of inflammatory cells, neutrophils, monocytes and lymphocytes, were determined using a coulter counter. The concentrations of NO and PGE2 were determined by Griess reagent (Sigma) and enzyme immunoassay (EIA) using a Correlate-EIA kit (Assay Designs, Ann Arbor, Ann ArboUSA), respectively.
In vitro RAW 264.7 cell culture, IFN-γ and LPS treatment greatly increased NO production (Figure 1). Pretreatment with fucoidan (1-320 µg/mL) did not affect the NO release from the macrophage cell line (Figure 1A). In comparison, CT extract (32-320 µg/mL) significantly suppressed NO production, indicative of an anti-inflammatory activity (Figure 1B).
Injection of carrageenan into mouse air pouch markedly increased the exudate volume within the pouch (Figure 2). Such increased exudation was attenuated by treatment with fucoidan (18 mg/kg) and CT extract (54 and 162 mg/kg). Notably, the combinational treatment of fucoidan and CT extract further decreased the exudates volume, compared to the individual effects, although higher dose combinations (54+162 mg/kg and 90+270 mg/kg) did not exhibit additional efficacy to the lowest dose combination (18+54 mg/kg) (Figure 2A). NO content in the pouch also drastically increased following carrageenan injection (Figure 2B). In comparison with the mild effect of fucoidan, CT extract markedly lowered the NO concentration. Combinational treatment of fucoidan and CT extract further decreased the NO level in a dose-dependent manner. In a similar manner, the increased PGE2 content was synergistically suppressed by the combinational therapy of fucoidan and CT extract (Figure 2C).
In parallel with the inflammatory mediators in the pouch, the numbers of inflammatory cells drastically increased by carrageenan injection: 37.8, 4.8 and 24.1 folds of control levels for neutrophils, monocytes and lymphocytes, respectively (Table 1). However, fucoidan and CT extract significantly reduced the inflammatory cell infiltration, in which fucoidan was somewhat superior to CT extract. Unexpectedly, no synergistic effect in the inflammatory cell infiltration was attained by co-administration of fucoidan and CT extract.
As inferred from the inflammatory cell infiltration in the exudates, the subcutaneous tissue surrounding air pouches exhibited severe inflammatory lesions (Figure 3). Enormously many cells in the thickened subcutaneous tissue exposed to carrageenan were observed (Figure 3B), compared with the near-normal features in control animals (Figure 3A). Such inflammatory lesions were remarkably attenuated by treatment with fucoidan (Figures 3C and 3D) or CT extract (Figures 3E and 3F). Notably, higher anti-inflammatory effects were obtained by combinational therapy with fucoidan and CT extract (Figures 3G-3I).
Using an in vivo carrageenan-induced air pouch inflammation model, injection of air to the backs of rodents induces proliferation of cells that stratify on the surface of the cavity to form a structure similar to the synovium. In this model, injection of carrageenan induced inflammatory cell infiltration and increased the exudate, which is indicative of vascular leakage. The air pouch serves as a reservoir of inflammatory cells and mediators that can be easily measured in the fluid that accumulates locally.
Among the diverse inflammatory mediators that can induce vascular permeability, it is well known that NO and PGE2 are major factors involved in the pathogenesis of many inflammatory diseases [24,25]. They are also known as mediators of pain induction and perception [10,11]. iNOS, which is expressed and activated in diverse cell types by stimulation with TNF-α and/or LPS during inflammation, produces high, persistent concentrations of NO [3,5-7]. NO is an important regulatory molecule in diverse physiological functions, such as vasodilatation leading to vascular leakage [8,9]. PGE2, which is generated from arachidonic acid via COXs, contributes to the development of many inflammatory diseases [26,27] and overproduction of PGE2 in response to various inflammatory stimulations is associated with up-regulation of COX-II level and progression of inflammation [28]. Therefore, TNF-α-NO and COX-II-PGE2 pathways have been considered as two main streams of inflammatory processes, which are blocked by inhibitors of iNOS (corticosteroids) and COX (NSAIDs), respectively [22,29].
In the present study, co-administration of fucoidan and CT extract reduced the exudate volume in the pouch, suggesting that they possess suppressive activity against vascular leakage (Figure 2A). In comparison with the individual effects of each fucoidan and CT extract, the combinational treatment further increased, implying that the two compounds action synergistically. Such synergistic effects between fucoidan and CT extract were supported by their activities on the NO and PGE2 levels in the exudates (Figures 3B and 2C).
Interestingly, however, it was confirmed that fucoidan does not directly inhibit the activation of macrophages in vitro assay (Figure 1A). Similarly, the inhibitory potential of fucoidan on the NO and PGE2 accumulation in vivo assay was relatively lower than that of CT extract (Figures 2B and 2C). Such an action mechanism of fucoidan is different from a direct inactivation of the cells by CT extract (Figure 1B). By comparison, fucoidan exerted a high efficacy on the inhibition of inflammatory cell infiltration in the pouches (Table 1). Actually, fucoidan was superior to CT extract in blocking the migration of neutrophils, monocytes and lymphocytes to the carrageenan-induced tissue injury sites. Interestingly, there was no synergistic anti-migratory effect, indicative of different action mechanisms between fucoidan and CT extract: i.e., fucoidan blocks infiltration of inflammatory cells, while CT extract inhibits activation of the cells.
Polymorphonuclear cells, especially neutrophils, are the main cellular components recruited to acute inflammatory sites induced by carrageenan. In addition, ensuing inflammatory cells such as macrophages and lymphocytes also play central roles in the inflammatory process by secreting cytokines [22,30,31]. When pretreated with fucoidan or CT extract, the number of white blood cells that migrated into the carrageenan-injected air pouches was markedly suppressed down. This result was consistent with an effect that would be obtained by corticosteroids such as dexamethasone, but not by NSAIDs including indomethacin [23,30]. Therefore, it is assumed that the fucoidan and CT extract have in part a property of steroids.
In addition to the blocking action on inflammatory cell chemotaxis, CT extract appeared to suppress the activation of macrophages. This was confirmed by its inhibitory actions on NO in vitro and in vivo, which is the major inflammatory mediator produced by macrophages. Thus, the effect of CT extract on macrophages might be due to a suppression of mRNA expression and/or a direct inhibition of iNOS activity in these cells.
PGE2 is one of the major inflammatory mediators, too. It increases in parallel with tissue edema, which is suppressed by NSAIDs, inhibitors of COX [21,22]. Carrageenan can increase PGE2 production by inducing COX-II mRNA and COX-II protein in the pouch exudate [32]. In this model, carrageenan significantly increased the amount of PGE2 and it was substantially suppressed by CT extract. It is interesting to note that this result was consistent with an effect that would be obtained by an NSAID indomethacin [23]. Therefore, it is inferred from the results that CT extract has additional property of NSAIDs. Taken together, it is believed that CT extract exerted both corticosteroid and NSAID-like effects in the carrageenan-induced inflammation model, and that fucoidan enhanced the anti-inflammatory effects of CT extract by blocking inflammatory cell infiltration.
Although defining the exact mechanism of action requires further study, a mixture of fucoidan and CT extract markedly attenuated inflammatory signs by blocking the TNF-α-NO and COX-II-PGE2 pathways in carrageenan-induced air pouch inflammation. These findings provide evidence that combinational treatment of fucoidan and CT extract might be a promising candidate for the relief of various types of inflammation that are responsive to corticosteroids or NSAIDs.
Figures and Tables
 | Figure 1Effects of fucoidan (A) and Cistanche tubulosa (CT) extract (B) on the nitric oxide production of RAW 264.7 cells stimulated with interferon-γ (IFN-γ, 10 U/mL) and lipopolysaccharide (LPS, 10 µg/mL). *Significantly different from vehicle control (P<0.05). #Significantly different from IFN-γ+LPS alone (P<0.05). |
 | Figure 2Effects of fucoidan and Cistanche tubulosa (CT) extract (in mg/kg) on the carrageenan-induced exudation and inflammatory responses in an air-pouch inflammation model. A: net exudate volume 6 hours after carrageenan injection. B: nitric oxide (NO) concentration in the exudates. C: prostaglandin E2 (PGE2) concentration in the exudates. *Significantly different from normal (Nor) control (P<0.05). #Significantly different from carrageenan (Car) alone (P<0.05). |
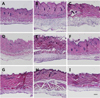 | Figure 3Representative microscopic findings of skin tissue of mice treated with carrageenan and/or fucoidan or Cistanche tubulosa (CT) extract. A, normal; B, carrageenan alone; C, fucoidan 18 mg/kg; D, fucoidan 54 mg/kg; E, CT extract 54 mg/kg; F, CT extract 162 mg/kg; G, fucoidan 18 mg/kg+CT extract 54 mg/kg; H, fucoidan 54 mg/kg+CT extract 162 mg/kg; I, fucoidan 90 mg/kg+CT extract 270 mg/kg. Bar=200 µm. |
Acknowledgments
This work was supported in part by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0031403).
References
1. Day R. Adverse reactions to TNF-α inhibitors in rheumatoid arthritis. Lancet. 2002. 359(9306):540–541.
2. Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996. 14:397–400.
3. Eigler A, Sinha B, Hartmann G, Endres S. Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol Today. 1997. 18(10):487–492.
4. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000. 51:245–270.
5. Adams V, Nehrhoff B, Späte U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G. Induction of iNOS expression in skeletal muscle by IL-1β and NFκB activation: an in vitro and in vivo study. Cardiovasc Res. 2002. 54(1):95–104.
6. Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J Exp Med. 1993. 177(6):1779–1784.
7. Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Kawai C. Purification of nitric oxide synthase from rat macrophage. J Biol Chem. 1991. 266(19):12544–12547.
8. Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988. 27(24):8706–8711.
9. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43(2):109–142.
10. Pang L, Hoult JR. Cytotoxicity to macrophages of tetrandrine, an anti-silicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem Pharmacol. 1997. 53(6):773–782.
11. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987. 30:103–114.
12. Lester RS, Knowles SR, Shear NH. The risks of systemic corticosteroid use. Dermatol Clin. 1998. 16(2):277–288.
13. Lichtenstein DR, Syngal S, Wolfe MM. Nonsteroidal antiinflammatory drugs and the gastrointestinal tract. The double-edged sword. Arthritis Rheum. 1995. 38(1):5–18.
14. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001. 286(8):954–959.
15. Feldman SC, Reynaldi S, Stortz CA, Cerezo AS, Damont EB. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine. 1999. 6(5):335–340.
16. Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol. 2010. 46(1):6–12.
17. Bojakowski K, Abramczyk P, Bojakowska M, Zwoliñska A, Przybylski J, Gaciong Z. Fucoidan improves the renal blood flow in the early stage of renal ischemia/reperfusion injury in the rat. J Physiol Pharmacol. 2001. 52(1):137–143.
18. Li N, Zhang Q, Song J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem Toxicol. 2005. 43(3):421–426.
19. Yamada P, Iijima R, Han J, Shigemori H, Yokota S, Isoda H. Inhibitory effect of acteoside isolated from Cistanche tubulosa on chemical mediator release and inflammatory cytokine production by RBL-2H3 and KU812 cells. Planta Med. 2010. 76(14):1512–1518.
20. Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, Choe SY, Kim YB. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a carrageenan-air pouch inflammation model. Exp Anim. 2009. 58(4):431–436.
21. Hevel JM, Marletta MA. Nitric-oxide synthase assays. Methods Enzymol. 1994. 233:250–258.
22. Romano M, Faggioni R, Sironi M, Sacco S, Echtenacher B, Di Santo E, Salmona M, Ghezzi P. Carrageenan induced acute inflammation in the mouse air pouch synovial model. Role of tumour necrosis factor. Mediators Inflamm. 1997. 6(1):32–38.
23. Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-air pouch inflammation. Br J Pharmacol. 1999. 126(5):1200–1204.
24. Guslandi M. Nitric oxide and inflammatory bowel diseases. Eur J Clin Invest. 1998. 28(11):904–907.
25. Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-α- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003. 111(6):821–831.
26. Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004. 63(9):901–910.
27. St-Onge M, Flamand N, Biarc J, Picard S, Bouchard L, Dussault AA, Laflamme C, James MJ, Caughey GE, Cleland LG, Borgeat P, Pouliot M. Characterization of prostaglandin E2 generation through the cyclooxygenase (COX)-2 pathway in human neutrophils. Biochim Biophys Acta. 2007. 1771(9):1235–1245.
28. Choi YH, Park HY. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J Biomed Sci. 2012. 19:31.
29. Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-air pouch inflammation. Br J Pharmacol. 1999. 126(5):1200–1204.
30. Oliveira de Melo J, da Conceição Torrado Truiti M, Muscará MN, Bolonheis SM, Dantas JA, Caparroz-Assef SM, Cuman RK, Bersani-Amado CA. Anti-inflammatory activity of crude extract and fractions of Nectandra falcifolia leaves. Biol Pharm Bull. 2006. 29(11):2241–2245.
31. Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006. 20(12):2118–2120.
32. Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is anti-inflammatory and nonulcerogenic. Proc Natl Acad Sci USA. 1994. 91(8):3228–3232.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download