Abstract
Adiaspiromycosis is caused by pulmonary infection with Emmonsia. Inhalated spores of Emmonsia cause asymptomatic infection to necrogranulomatous pneumonia, depending on the burden of adiaspore and host immunity. For disease monitoring of wild rodents captured on Jeju Island in Korea, we examined the lung tissue of wild rodents histopathologically. Spores composed of thick three-layered walls were found following histopathological examination and were diagnosed as adiaspiromycosis. Adiaspiromycosis has been found in mammals in many parts of the world. To our knowledge, this is the first report of adiaspiromycosis of an Apodemus agrarius captured in Korea.
Adiaspiromycosis is a pulmonary fungal infection caused by the dimorphic fungi, Emmonsia parva or Emmonsia crescens [1]. Large globose, thick-walled, non-proliferating structures called adiaspore [2] are seen in infected tissue. The term adiaspore was derived from the Greek verb speirein for scattering, with adia being a negative, so adiaspiromycosis describes an infection in which there is no multiplication or dissemination of the fungus from the original site [3].
Emmonsia species are ubiquitous filamentous fungi isolated commonly from soil [1]. Emmonsia crescens (Chrysosporium parvum var. crescens) has been isolated from over 96 species of animals as well as soil worldwide, whereas Emmonsia parva (Chrysosporium parvum) has been isolated from relatively few species of animals in narrow geographical ranges [2].
Emmonsia crescens is the main causative agent of adiaspiromycosis in mainland Europe and the UK, whereas Emmonsia parva is widespread in certain exothermic regions, including Central Asia, Africa and parts of the America [4]. The first human case of adiaspiromycosis due to Emmonsia crescens was reported in France in 1960 [4] and human pulmonary adiaspiromycosis has been reported in the literature from multiple countries including Russia, Germany, the Czech Republic, Guatemala, Brazil and the United States [5,6]. Infection of wild life has been reported in squirrels in Canada [7], Eurasian otters (Lutra lutra) in England [8], European beavers in Sweden [9], raccoons (Procyon lotor) [10], bullfrogs (Rana catesbeiana) in US [11] and European hedgehogs (Erinaceus europaeus) in Portugal [12].
Inhalation of soil dust containing spores of Emmonsia is the main route of infection [13]. The infection's pathological effects range from asymptomatic infection to necrogranulomatous pneumonia and death, depending on the burden of adiaspore and host immunocompetence [14]. Adiapiromycosis patients have a chronic history of progressive dyspnea, nonproduction cough, fatigue, low-grade fever and, less frequently, with hemophysis, pain, chills, malaise, weight loss and auscultatory crackles [14,15,16,17]. In addition to the pulmonary organ, Emmonsia crescens can cause cutaneous adiaspiromycosis and associated acute conjunctivitis [5]. In many of the reported cases, Emmonsia crescens infection was related to play in the surroundings of an animal burrow, which may have played the role of a reservoir, and other outdoor activities [18].
Inhalated E. crescens develops into large, thick-walled spherules called adiaspores, measuring as much as 700 µm, and originating from minute (2~4 µm) subglobose conidia. Infectious E. crescens cannot germinate at the elevated temperatures of the host, and instead increases in volume to form thick-walled, non-replicating adiaspores that elicit extensive granulomatous reaction [4]. Expanding adiaspores cause collapse of the adjacent alveoli and respiratory distress or even failure [19]. Clinical signs are not significant in rodents [9]. However, adiaspore can form small or more extensive foci on the surface of the liver and spleen in experimental infection of adiaspiromycosis with intraperitoneal injection in wild rodents [20].
Diagnosis of adiaspiromycosis is difficult because the fungus is not easily cultured. Histological observation of characteristic adiaspores with light microscopy [13] in lung tissue specimen such as human biopsy samples [18] has been performed. However, Emmonsia parva and Emmonsia crescens are morphologically indistinguishable in their mycelial phases, which makes differential diagnosis difficult. E. crescens produces multinucleate adiaspores at temperatures above 30-37℃ (depending on the isolate) [21] which routinely reach diameters in excess of 500 µm [4], whereas Emmonsia parva isolates produce adiaspores that are mononucleate and substantially smaller (20-40 µm in diameter) only at temperatures approaching or in excess of 40℃ [4].
During the surveillance for disease in wild rodents captured on Jeju Island, Korea, we detected multiple circular spore-like materials surrounded by granulomatous inflammation following histopathological examination. The Apodemus agrarius was female and captured on Jeju Island (N 33°20'33.8", E126°20'12.1") in August 2010. The body weight was 37.3 g and the body length was 107.84 mm. On that day necropsy was performed on the wild rodent. In the necropsy, specific gross lesions were not detected; brain, heart, lung, liver, spleen, stomach, intestine, and kidney were collected and fixed in 10% buffered formalin for 48 hours. After alcohol-xylene processing and embedding with paraffin, 4 µm thick sections were stained by haematoxylin and eosin and observed with a light microscope.
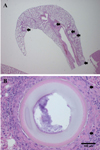
Round structures were randomly scattered throughout the entire lung field (Figure 1A). These structures were located in alveolar space, had thick trilaminar walls consisting of a basophilic outer-layer (3.4 µm in diameter 227 µm structure), an eosinophilic mid-layer (11 µm in diameter 227 µm structure) and a pale colored inner-layer (43 µm in diameter 227µm structure) and a basophilic granular retiform part in the center (approximately 119 µm in diameter 227 µm structure). The diameter of the structures was 195-500 µm (mean 263 µm). The structures mildly compressed the surrounding tissues that were encapsulated by multinucleated giant cells, macrophages (epitheloid cells) and lymphocytes (Figure 1B).
We diagnosed the lesion found in the lung as adiaspiromycosys by Emmonisa sp. infection based on the shape, stained appearance, and lesion. That the structures have thick outer walls and inner basophilic parts are adiaspores of fungus and those are the cause of the granulomatous lesion in the lung. A total of 12 wild rodents (Apodemus agrarius) were captured in this surveillance. Only one such case was detected. The entirety of the lung tissue was not examined microscopically, only part of the lungs was inspected. Therefore, many infected cases could have been missed. And unfortunately, we could not identify whether the agent was Emmonsia crescen or Emmonsia parvum. But it is more likely that it was Emmonsia crescen, based on the difference in their distribution in the world [4]. The number of recorded infections of wild rodents is low. To our knowledge, this is the first report of adiaspiromycosis of wild rodents captured in Korea.
Figures and Tables
Figure 1
Adiaspores of Emmonsia sp. in the lung parenchyma of an Apodemus agrarius. (A) Round structures (black arrows) scattered in the lung. (B) Adiaspore was located in alveolar space encapsulated by granulomatous inflammatory lesion. Macrophages and Langerhans giant cells infiltrated in the surrounding granulomatous tissue. The spores have three layers in their walls and basophilc granular structures in their inner parts. Haematoxylin and Eosin stain. Bar=100 µm
Acknowledgments
This study was supported by the research fund of the National Institute of Environmental Research and Research Institute for Verterinary Science, Seoul National University.
References
1. Chantrey JC, Borman AM, Johnson EM, Kipar A. Emmonsia crescens infection in a British water vole (Arvicola terrestris). Med Mycol. 2006. 44(4):375–378.
2. Sigler L. Ajellomyces crescens sp. nov., taxonomy of Emmonsia spp., and relatedness with Blastomyces dermatitidis (teleomorph Ajellomyces dermatitidis). J Med Vet Mycol. 1996. 34(5):303–314.
3. Emmons CW, Jellison WL. Emmonsia crescens sp. n. and adiaspiromycosis (haplomycosis) in mammals. Ann N Y Acad Sci. 1960. 89:91–101.
4. Borman AM, Simpson VR, Palmer MD, Linton CJ, Johnson EM. Adiaspiromycosis due to Emmonsia crescens is widespread in native British mammals. Mycopathologia. 2009. 168(4):153–163.
5. Mendes MO, Moraes MA, Renoiner EI, Dantas MH, Lanzieri TM, Fonseca CF, Luna EJ, Hatch DL. Acute conjunctivitis with episcleritis and anterior uveitis linked to adiaspiromycosis and freshwater sponges, Amazon region, Brazil. Emerg Infect Dis. 2009. 15(4):633–639.
6. Wellinghausen N, Kern WV, Haase G, Rozdzinski E, Kern P, Marre R, Essig A, Hetzel J, Hetzel M. Chronic granulomatous lung infection caused by the dimorphic fungus Emmonsia sp. Int J Med Microbiol. 2003. 293(6):441–445.
7. Leighton FA, Wobeser G. The prevalence of adiaspiromycosis in three sympatric species of ground squirrels. J Wildl Dis. 1978. 14(3):362–365.
8. Simpson VR, Gavier-Widen D. Fatal adiaspiromycosis in a wild Eurasian otter (Lutra lutra). Vet Rec. 2000. 147(9):239–241.
9. Mörner T, Avenäs A, Mattsson R. Adiaspiromycosis in a European beaver from Sweden. J Wildl Dis. 1999. 35(2):367–370.
10. Hamir AN. Pulmonary adiaspiromycosis in raccoons (Procyon lotor) from Oregon. J Vet Diagn Invest. 1999. 11(6):565–567.
11. Hill JE, Parnell PG. Adiaspiromycosis in bullfrogs (Rana catesbeiana). J Vet Diagn Invest. 1996. 8(4):496–497.
12. Seixas F, Travassos P, Pinto ML, Pires I, Pires MA. Pulmonary adiaspiromycosis in a European hedgehog (Erinaceus europaeus) in Portugal. Vet Rec. 2006. 158(8):274–275.
13. Pusterla N, Pesavento PA, Leutenegger CM, Hay J, Lowenstine LJ, Durando MM, Magdesian KG. Disseminated pulmonary adiaspiromycosis caused by Emmonsia crescens in a horse. Equine Vet J. 2002. 34(7):749–752.
14. Barbas Filho JV, Amato MB, Deheinzelin D, Saldiva PH, de Carvalho CR. Respiratory failure caused by adiaspiromycosis. Chest. 1990. 97(5):1171–1175.
15. England DM, Hochholzer L. Adiaspiromycosis: an unusual fungal infection of the lung. Report of 11 cases. Am J Surg Pathol. 1993. 17(9):876–886.
16. Nuorva K, Pitkänen R, Issakainen J, Huttunen NP, Juhola M. Pulmonary adiaspiromycosis in a two year old girl. J Clin Pathol. 1997. 50(1):82–85.
17. Peres LC, Figueiredo F, Peinado M, Soares FA. Fulminant disseminated pulmonary adiaspiromycosis in humans. Am J Trop Med Hyg. 1992. 46(2):146–150.
18. Dot JM, Debourgogne A, Champigneulle J, Salles Y, Brizion M, Puyhardy JM, Collomb J, Plénat F, Machouart M. Molecular diagnosis of disseminated adiaspiromycosis due to Emmonsia crescens. J Clin Microbiol. 2009. 47(4):1269–1273.
19. Hubálek Z. Emmonsiosis of wild rodents and insectivores in Czechland. J Wildl Dis. 1999. 35(2):243–249.
20. Krivanec K, Otcenásek M, Prokopic J. Experimental adiaspiromycosis of the common vole (Microtus arvalis) and other small wild mammals after intraperitoneal inoculation. Mycopathol Mycol Appl. 1974. 53(1):133–140.
21. Boisseau-Lebreuil MT. In vitro formation of adiospores in 10 strains of Emmonsia cresens, the fungal agent of adiospiromycosis. C R Seances Soc Biol Fil. 1975. 169(4):1057–1061.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download