Abstract
Overweight and obesity are usually related with high fat and calorie intake, and seriously causative of lifestyle-related diseases such as cardiovascular disorders, arteriosclerosis, and colon cancer. In this study, we propose a novel dietary therapy against overweight and obesity using mixtures of protamine and chitooligosaccharide (COS), which are known to interrupt the lipid metabolism in the body. Protamine is a dietary protein originated from salmon reproductive organ, and COS is an oligosaccharide made from chitin or chitosan by chemical or enzymatic hydrolysis. In the enzyme activity analysis in vitro, protamine and COS strongly suppressed the activity of pancreatic lipase, which is the primary enzyme for the digestion and absorption of lipids in the intestine. In in vivo animal test, the mixtures of protamine and COS significantly reduced the serum levels of triglyceride (TG), total cholesterol (T-CHO), and low density lipoprotein-cholesterol (LDLC) and inhibited the accumulation of lipids in liver tissue of Sprague Dawley (SD) rats fed high fat diets. On the other hand, they increased fecal TG and T-CHO contents. From these alterations in lipid metabolism, we verified that protamine and COS mixtures could effectively interrupt the digestion and absorption of dietary lipids in the body by inhibiting pancreatic lipase activity. In addition, protamine and COS mixtures increased the serum level of high density lipoprotein-cholesterol (HDLC), responsible for removing cholesterol from cells and protecting atherosclerosis, and therefore decreased the potential risks of cardiovascular diseases by lowering values of the atherogenic index (AI) and cardiac risk factor (CRF). Taken together, we suggest protamine and COS mixtures as a prominent dietary therapy for the prevention of overweight, obesity, and further cardiovascular diseases related with hyperlipidemia.
In the aftermath of industrialization, eating habits of South Korea have changed to Western diets, including bread, meat, liquor, and fast food, which sometimes can be related with high fat and calorie intake. Recently, a frequent 'get-together', one of distinctive sociocultural events in South Korea, also causes overweight by excessive drinking and meat-eating. For these reasons, the number of overweighed and obese people has been increasing in the last few decades. The World Health Organization (WHO) claims that 45% of Korean men and 54% of women are overweighed in 2005, and the percentages are expected to increase to 66% and 67%, respectively by 2015. The overweight and obesity are often involved with cardiovascular disorders, arteriosclerosis, colon cancer, and other diseases [1]. Some studies have shown that dietary therapy is important and could be considered as the first choice in the treatment of disorders resulting from overweight and obesity [2]. In the present study, we propose the dietary mixtures of protamine and chitooligosaccharide (COS) with the prominent improvement effect on lipid metabolism as a novel strategy against overweight due to high fat ingestion.
Protamine is a protein originated from salmon sperm. It has a low molecular weight of around 4,000-5,000 and functions in protecting DNA damage and restricting fat absorption in the intestine [3]. In addition, protamine is widely used as a natural food preservative due to strong antibacterial effect [4]. In terms of lipid metabolism, protamine has been proved to be effective in reducing weight gain and body fat accumulation through the inhibition of dietary fat absorption, because it strongly inhibits the hydrolysis of triglycerides, which is a process needed for absorption of lipid into the intestine [5]. Another candidate for dietary therapy, COS, is an oligosaccharide made from chitin or chitosan by chemical or enzymatic hydrolysis. Polysaccharides, chitin or chitosan, have limited application as nutrient source due to their insolubility and high viscosity. On the other hand, as COS has low molecular weight, good solubility, and low viscosity [6,7], it can be diversely used in many formulations. Several recent studies have identified various health benefits of COS. For example, COS has shown to have anti-bacterial and anti-fungal activities and to enhance immune function [8,9]. In addition, it restores healthy blood pressure, reduces cholesterol, and prevents alcoholic liver disease [10]. Especially, COS has also been shown to reduce the triglyceride level in obese diabetic mice [11,12]. and COS was evaluated as an effective dietary supplement for lowering cholesterol level in healthy men [13]. Therefore, COS has recently attracted highly attention as a new biomedical.
This study was conducted to examine the possibility of the protamine and COS mixtures as a dietary therapy against overweight and obesity focusing on lipid metabolism. We evaluated whether these mixtures suppress the pancreatic lipase activity in vitro and further restraint the lipid metabolism and absorption in vivo. Male Sprague Dawley (SD) rats were used in the in vivo animal test and the contents of TG, T-CHO, HDLC, and LDLC in serum or feces were measured after the dietary administration of the protamine and COS mixtures with high fat diet to rats. Also, the lipid accumulation in liver tissue was apparently identified by histochemical analysis.
Salmon protamine (hydrochloride salt, Maruha Nichiro Foods, Japan) and COS (CNA Biotech, Cheongwon, Republic of Korea) were provided by LG Household & Health Care Research (Deajeon, Republic of Korea). Protamine and COS have a purity of 98% and 88%, respectively. All other chemicals used were of reagent grade.
The pancreatic lipase activity was measured using 4-methylumbelliferyl oleate (4-MU oleate) as a substrate. The samples of protamine or COS were prepared at various concentrations dissolved in distilled water. The concentrations of protamine samples were 0, 2, 4, 8, 16, 32, 64, and 100 µg/mL, and the concentrations of COS samples were 0, 5, 25, 50, 75, 100, 200, and 300 µg/mL. Firstly, 5 µL of each sample was put in 96-wells plate and then added with 50 µL of 0.1 mM 4-MU solution buffer (13 mM Tris-HCl, 150 mM NaCl, and 1.3 mM CaCl2, pH8.0). Twenty five µL of the porcine pancreatic lipase solution dissolved in the above buffer (50 U/mL) was then added to start the enzyme reaction. After incubation at 36℃ for 30 min, 0.1 mL of 0.1 M sodium citrate (pH 4.2) was added to stop the reaction [14,15]. The amount of 4-methylumbelliferone released by lipase was measured at 450 nm using an ELISA-Reader (VERSA man, Molecular Devices, Sunnyvale, CA, USA).
Healthy male SD-rats were purchased from Central Lab Animal, Inc. (Seoul, Republic of Korea). Seven-week-old male SD-rats were housed in a conventionalfacility at the Laboratory Animal Research Center of Chungbuk National University (Cheongju, Republic of Korea). The animals were maintained in a room with constant temperature of 22±℃, relative humidity of 55±10%, and 12 h light/dark cycle, and fed standard rodent chow. Rats were allowed to acclimate for 1 week after arrival. All animals were used for the in vivo experiments in accordance with the approved institutional guidelines of Chungbuk National University. The experiment was allowed to conduct by Institution Animal Care IACUC and use committee of Chungbuk National University (CBNUA-363-11-01).
A high-fat diet was prepared as corn oil suspension by mixing the 6 mL of corn oil (Sigma-Aldrich, St. Louis, MO, USA), 80 mg of cholic acid (Sigma-Aldrich), 2 mg of cholesteryl oleate (Sigma-Aldrich), and 1 mg of margarine (Seoul Milk Ltd., Seoul, Republic of Korea) in 6 mL of distilled water [16]. The mixture diets of protamine and COS were made by adding protamine and COS at various ratios into the above corn oil suspension. The PO2.1 mixture contained protamine 2.1 mg/kg body weight (b.w.) and COS 25 mg/kg b.w. in corn oil suspension (protamine:COS=1:12), PO4.2 mixture protamine 4.2 mg/kg b.w. and COS 25 mg/kg b.w. (protamine:COS=1:6), and PO8.3 mixture protamine 8.3 mg/kg b.w. and COS 25 mg/kg b.w. (protamine: COS=1:3).
Male SD-rats, weighing 296.86±2.46 g, were divided into four groups and 3 mL of experimental diet was administered once to each rat via a Zonde needle. The vehicle group (n=6) was treated only with corn oil suspension. Other experimental groups (each group, n=5) were administered with the mixture diets of protamine and COS added in corn oil suspension. Before the oral administration of experimental diets, all rats were starved except water for 18 h and then blood samples were collected from the tail vein (0 h). After the administration, blood samples were collected at 3, 9, and 24 h. The feces were also collected from each group at 0, 3, 9, and 24 h and stored at -20℃ until analysis.
Blood samples (0.5~0.7 mL) were left at room temperature for 1 h, and then serum was prepared by centrifugation at 4℃, 3,000 rpm for 20 min and then stored at -20℃. Serum analysis was conducted by HITACHI Clinical Analyzer 7080 (Hitachi Korea Ltd., Seoul, Korea) to measure the serum concentrations of various lipids including TG, T-CHO, HDLC, and LDLC. The AI and CRF were converted from serum HDLC and T-CHO concentrations. The AI was calculated as (T-CHO-HDLC) / HDLC. The CRF was calculated as (T-CHO/ HDLC). Next, the collected feces were analyzed for fecal lipids. The fecal crude fat was extracted by Rose-Gottlieb method [17]. Five g of the feces was put into Mojonnier fat-extractor, added with 6 mL NH4OH (OCI Company Ltd., Ulsan, Republic of Korea), and then left for 3 min. This mixture was mixed with 12 mL of 95% alcohol (OCI Company Ltd.), 25 mL of ether (OCI Company Ltd.), and 25 mL of petroleum ether (OCI Company Ltd.), and then left at room temperature for 1~2 h. Finally, fecal crude fat was collected by withdrawing the supernatant consisted of ether phase at 75℃ and analyzed for TG and T-CHO. Fecal TG was measured by triglyceride assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer's method. Fecal T-CHO was analyzed by Enzychrome cholesterol assay kit (BioAssay Systems, Hayward, CA, USA) according to the manufacturer's method.
After 24 h of oral administration with experimental diets, the liver tissues were harvested from the sacrificed rats and then immediately frozen up in -80℃ deep freezer. Frozen liver tissues were cryo-sectioned 6-µm thick, fixed in 10% formalin solution (OCI Company Ltd.) at 4℃ for 5 min, and then rinsed 3 times with distilled water. 5% Oil-red-O working solution was prepared by dissolving Oil-red-O powder (Sigma-Aldrich) in Propylene Glycol (OCI Company Ltd.) and used to stain the sectioned tissues according to the standard methods of manufacturer's. Counter staining was conducted with the hematoxylin (Sigma-Aldrich) and then mounted with glycerin (OCI Company Ltd.). The lipid containing cells were detected as the red spot using a light microscope (BX51 U-LH100HGWIG, Olympus, Tokyo, Japan; ×40, ×200, and ×400 magnification).
All data were analyzed with GraphPad Prism software (San Diego, CA, USA) [18,19]. The in vitro data are presented as the mean±SD and in vivo data as the mean±SEM. Statistical analysis was performed using a one-way ANOVA followed by Dunnett's multiple comparison test. P-values <0.05 were considered to be statistically significant.
Protamine and COS significantly inhibited pancreatic lipase activity in vitro. The enzyme activity was decreased by 29% at 2 µg/mL of protamine, and by 93% at 32 µg/mL of protamine, respectively (Figure 1A). The COS also considerably inhibited pancreatic lipase activity, which was suppressed by 87% at 5 µg/mL of COS as shown in Figure 1B.
The contents of various types of lipid in serum including TG, T-CHO, HDLC, and LDLC were analyzed from the rats administered with experimental diets. The serum TG concentrations in vehicle group rapidly increased after the intake of high fat diet. But, PO2.1, PO4.2, and PO8.3 groups significantly inhibited the serum TG concentrations by 51, 54, and 47%, respectively, at 3 h, and 37, 56, and 57%, respectively, at 9 h compared with vehicle group (Figure 2A). Serum T-CHO concentration also effectively decreased by protamine and COS mixtures. PO2.1, PO4.2, and PO8.3 groups significantly decreased serum T-CHO by 30, 36, and 22%, respectively, at 3 h, and 34, 36, and 21%, respectively, at 9 h compared with vehicle group as seen in Figure 2B. Serum HDLC concentrations of PO 2.1, PO 4.2, and PO 8.3 groups were increased by 24, 39, and 33%, compared with vehicle group at 24 h (Figure 2C). Serum LDLC concentrations of PO 2.1, PO 4.2, and PO 8.3 groups were decreased by 39, 40, and 32%, respectively, compared with vehicle group at 24 h as shown in Figure 2D.
The AI and CRF of rats fed protamine and COS mixtures were significantly decreased compared with vehicle group as demonstrated in Table 1. The CRF of PO 4.2 group was decreased by 76% and AI of PO 2.1 group was decreased by 45%.
Protamine and COS mixture diets significantly increased the TG and T-CHO contents in feces compared to high fat diet administered to vehicle group. The fecal TG concentration of PO 2.1 group was highly increased by lapse of time up to 91% at 24 h (Figure 3A). The fecal T-CHO concentration of PO 4.2 group was increased up to 68% at 3 h. The PO 2.1 group gradually increased the fecal T-CHO and the PO 8.3 group effectively increased the fecal T-CHO only at 9 h as seen in Figure 3B.
The lipids accumulated in rat liver tissues obtained from each experimental group were detected as red spots in the histological analysis by Oil-red-O staining. The red spots show the lipid components which were digested from TG and T-CHO in high-fat diet by pancreatic lipase, absorbed into the intestine, and then accumulated in the liver. The red spot areas stained with lipids were observed in vehicle group, but not in protamine and COS mixture groups as demonstrated Figure 4.
Protamine from salmon reproductive organ is a dietary protein rich in the basic amino acid arginine and is known to be effective in reducing fat accumulation in the body through the inhibition of lipid absorption like other dietary proteins including soy and fish proteins [20,21]. COS is an oligosaccharide obtained from polysaccharides, chitin or chitosan, by chemical or enzymatic hydrolysis and has been recently evaluated as an effective dietary supplement for lowering cholesterol level in healthy men and animals [11,13]. In this study, we provide the mixtures of protamine and COS, two prominent candidates related with lipid metabolism, as a novel dietary therapy for overweight and obesity. We evaluated the inhibitory effect on lipid metabolism of these mixtures in vivo through the analysis on the serum and fecal lipid contents and lipid accumulation in liver tissues within 24 h after the administration of high fat diets to SD rats.
The mixture of protamine and COS significantly decreased the serum TG concentration, which was rapidly increased in a short time (3 h) after the intake of high fat diets. The serum TG concentration was decreased by about 50% by these mixtures at 3 h. For serum cholesterols, T-CHO and LDLC concentrations were also increased by high fat diets, while protamine and COS mixtures effectively decreased their levels at 3 h. On the other hand, HDLC was increased by these mixtures within 24 h compared with high fat diets. Unlike the serum level, the TG concentration in the feces was considerably increased by these mixtures in the same period time. The T-CHO concentration in feces also showed the tendency to increase. These contrary changes in lipid contents in serum and feces can be explained by the lipid metabolism altered by protamine and COS. Protamine and COS strongly suppressed the enzyme activity of pancreatic lipase at the very low concentration by the enzyme activity analysis. Pancreatic lipase is the primary lipase secreted from the pancreas, that hydrolyzes dietary lipids in the digestive system, converting TG substrates found in ingested oils to monoglycerides and free fatty acids. In the intestine, monoglycerides and free fatty acids are subsequently moved to enterocytes, cells lining the intestines and then absorbed. Therefore, pancreatic lipase is an important enzyme for lipid absorption in the intestine [22,23]. By strongly inhibiting the pancreatic lipase activity, protamine and COS can effectively restrain the lipid absorption in the intestine and induce the excretion of lipid outside the body. The inhibitory effect on pancreatic lipase by COS was first demonstrated in the present study.
The apparent evidence for the hindered lipid accumulation in the body by protamine and COS mixtures was presented in the histological analysis, in which the liver tissues obtained from rats fed high fat diets showed a great deal of red spots stained with Oil-red-O working solution, but not much spots were found in rats administered with protamine and COS mixtures. In addition to stimulating the excretion of dietary lipids, protamine and COS showed to improve the cholesterol status in the body. They decreased the serum T-CHO and increased the fecal T-CHO, and increased the HDLC and decreased the LDLC in serum. According to the lipid hypothesis, abnormal cholesterol levels are strongly associated with cardiovascular diseases because they promote atheroma development in arteries (atherosclerosis), which is more accurately caused by the condition of higher concentrations of LDLC and lower concentrations of functional HDLC [24,25]. It's because that LDLC particles have been linked to atheroma formation, and on the other hand, HDLC can remove cholesterol from cells and atheroma and offer protection to the disease. So, LDLC is sometimes referred to as "bad cholesterol" and HDLC "good cholesterol". As a result, protamine and COS mixtures decreased the potential risks of cardiovascular diseases by lowering values of the AI and CRF, which are represented in terms of T-CHO and HDLC levels in serum. A future study is required to elucidate the mechanisms underlying the improvement effect on cholesterol levels by protamine and COS.
In conclusion, the mixtures of protamine and COS effectively reduced the serum TG, T-CHO, and LDLC levels rapidly increased by high fat diets, and enhanced the serum HDLC level. In addition, these mixtures promoted the excretion of dietary lipids by blocking digestion, absorption, and accumulation in the body through their inhibition effect on the pancreatic lipase. With these alterations in lipid metabolism, we suggest the mixtures of protamine and COS as a prominent dietary therapy for the prevention of overweight, obesity, and further cardiovascular diseases related with hyperlipidemia.
Figures and Tables
 | Figure 1Pancreatic lipase activity. (A) The pancreatic lipase activity was inhibited by protamine. Protamine was used at the concentrations of 0, 2, 4, 8, 16, 32, 64, and 100 µg/mL. (B) The pancreatic lipase activity was inhibited by chitooligosaccharide (COS).The COS was used at the concentrations of 0, 5, 25, 50, 75, 100, 150, and 300 µg/mL. Values represent the mean±SD. *P<0.05 (Dunnett's multiple comparison test). |
 | Figure 2Serum lipid analysis. (A) Triglyceride in serum was measured after the oral administration of various mixtures of protamine and chitooligosaccharide (COS) in corn oil suspension. (B) Total-cholesterol in serum (C) High density lipoprotein-cholesterol (HDLC) (D) Low density lipoprotein-cholesterol (LDLC).The ratios of protamine and COS in the mixtures were 1:3, 1:6, and 1:12. The PO2.1 group was treated with 2.1 mg/kg body weight (b.w.) of protamine and 25 mg/kg b.w. of COS in corn oil suspension. The PO4.2 group was treated with 4.2 mg/kg b.w. of protamine and 25 mg/kg b.w. of COS in corn oil suspension. The PO8.3 group was treated with 8.3 mg/kg b.w of protamine and 25 mg/kg b.w of COS in corn oil suspension. Values represent the mean±SEM. *P<0.05 (Dunnett's multiple comparison test). |
 | Figure 3Fecal lipid analysis. (A) Triglyceride concentration in feces. (B) Total-cholesterol concentration in feces. The PO2.1 group was treated with 2.1 mg/kg body weight (b.w.) of protamine and 25 mg/kg b.w. of COS in corn oil suspension. The PO4.2 group was treated with 4.2 mg/kg b.w. of protamine and 25 mg/kg b.w. of COS in corn oil suspension. The PO8.3 group was treated with 8.3 mg/kg b.w. of protamine and 25 mg/kg b.w. of COS in corn oil suspension. Values represent the mean±SEM. *P<0.05 (Dunnett's multiple comparison test). |
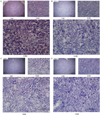 | Figure 4Histological analysis on liver tissues. (A) The liver tissue of vehicle group. (B) The PO2.1 group. (C) The PO4.2 group. (D) The PO8.3 group. After 24 h of oral ministration of protamine and COS mixtures, we harvested the liver tissues from rats. Frozen liver tissues were cryo-sectioned, cut 6-ìm thick, and stained with the 5% Oil-red-O working solution by standard methods of manufacturer's. Counter staining was conducted with the hematoxylin and then mounted with glycerin. The lipid containing cells are shown the red spots under a light microscope (BX51 U-LH100HGWIG, Olympus; ×40, ×200, and ×400 magnification). |
Table 1
Contents of CRF and AI in serum of SD-rats fed a high-fat diet containing various mixtures of protamine and chitooligosaccharide

1)Vehicle: high-fat diet, PO2.1: high-fat diet+protamine 2.1 mg/kg body weight (b.w.)+chitooligosaccharide (COS) 25 mg/kg b.w., PO4.2: high-fat diet+protamine 4.2 mg/kg b.w.+COS 25 mg/kg b.w., PO8.3: high-fat diet+protamine 8.3 mg/kg b.w.+COS 25 mg/kg b.w.
2)CRF (cardiac risk index): total cholesterol (T-CHO)/high density lipoprotein cholesterol (HDLC)
3)AI (artherogenic index): (T-CHO-HDLC)/HDLC
4)The results are mean±SEM of 3 rats per each group. *P<0.05 (Dunnett's multiple comparison test).
Acknowledgments
This work was supported by the research grant of the Chungbuk National University in 2011.
References
1. Pokorski R. Effect of increasing body weight on morbidity and mortality in South Korea. J Insur Med. 2011. 42(2-4):78–84.
2. Bae JM, Yang YJ, Li ZM, Ahn YO. Low cholesterol is associated with mortality from cardiovascular diseases: a dynamic cohort study in Korean adults. J Korean Med Sci. 2012. 27(1):58–63.
3. Hosomi R, Fukunaga K, Arai H, Kanda S, Nishiyama T, Yoshida M. Effect of dietary protamine on lipid metabolism in rats. Nutr Res Pract. 2010. 4(6):462–469.
4. Aspedon A, Groisman EA. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology. 1996. 142(Pt 12):3389–3397.
5. Duarte-Vázquez MA, García-Padilla S, Olvera-Ochoa L, González-Romero KE, Acosta-Iñiguez J, De la Cruz-Cordero R, Rosado JL. Effect of protamine in obesity induced by high-fat diets in rats. Int J Obes (Lond). 2009. 33(6):687–692.
6. Chae SY, Jang MK, Nah JW. Influence of molecular weight on oral absorption of water soluble chitosans. J Control Release. 2005. 102(2):383–394.
7. Zhou TX, Chen YJ, Yoo JS, Huang Y, Lee JH, Jang HD, Shin SO, Kim HJ, Cho JH, Kim IH. Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens. Poult Sci. 2009. 88(3):593–600.
8. Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH, Kim CY. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int J Antimicrob Agents. 2001. 18(6):553–557.
9. Shon YH, Nam KS. Inhibition of polyamine biosynthesis in Acanthamoeba castellanii and 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity by chitosanoligosaccharide. Biotechnol Lett. 2003. 25(9):701–704.
10. Cho SY, Lee JH, Song MJ, Park PJ, Shin ES, Sohn JH, Seo DB, Lim KM, Kim WG, Lee SJ. Effects of chitooligosaccharide lactate salt on sleep deprivation-induced fatigue in mice. Biol Pharm Bull. 2010. 33(7):1128–1132.
11. Hayashi K, Ito M. Antidiabetic action of low molecular weight chitosan in genetically obese diabetic KK-Ay mice. Biol Pharm Bull. 2002. 25(2):188–192.
12. Tang ZR, Yin YL, Nyachoti CM, Huang RL, Li TJ, Yang C, Yang XJ, Gong J, Peng J, Qi DS, Xing JJ, Sun ZH, Fan MZ. Effect of dietary supplementation of chitosan and galacto-mannan-oligosaccharide on serum parameters and the insulin-like growth factor-I mRNA expression in early-weaned piglets. Domest Anim Endocrinol. 2005. 28(4):430–441.
13. Choi CR, Kim EK, Kim YS, Je JY, An SH, Lee JD, Wang JH, Ki SS, Jeon BT, Moon SH, Park PJ. Chitooligosaccharides decreases plasma lipid levels in healthy men. Int J Food Sci Nutr. 2012. 63(1):103–106.
14. Nakai M, Fukui Y, Asami S, Toyoda-Ono Y, Iwashita T, Shibata H, Mitsunaga T, Hashimoto F, Kiso Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J Agric Food Chem. 2005. 53(11):4593–4598.
15. Zhang J, Kang MJ, Kim MJ, Kim ME, Song JH, Lee YM, Kim JI. Pancreatic lipase inhibitory activity of taraxacum officinale in vitro and in vivo. Nutr Res Pract. 2008. 2(4):200–203.
16. Tsujita T, Matsuura Y, Okuda H. Studies on the inhibition of pancreatic and carboxylester lipases by protamine. J Lipid Res. 1996. 37(7):1481–1487.
17. Gors S, Kucia M, Langhammer M, Junghans P, Metges CC. Technical note: Milk composition in mice--methodological aspects and effects of mouse strain and lactation day. J Dairy Sci. 2009. 92(2):632–637.
18. Hwang KA, Park SH, Yi BR, Choi KC. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol a using microarray analysis. Lab Anim Res. 2011. 27(2):99–107.
19. Yi BR, Kang NH, Hwang KA, Kim SU, Jeung EB, Choi KC. Antitumor therapeutic effects of cytosine deaminase and interferon-β against endometrial cancer cells using genetically engineered stem cells in vitro. Anticancer Res. 2011. 31(9):2853–2861.
20. Hosomi R, Fukunaga K, Arai H, Nishiyama T, Yoshida M. Effects of dietary fish protein on serum and liver lipid concentrations in rats and the expression of hepatic genes involved in lipid metabolism. J Agric Food Chem. 2009. 57(19):9256–9262.
21. Moriyama T, Kishimoto K, Nagai K, Urade R, Ogawa T, Utsumi S, Maruyama N, Maebuchi M. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci Biotechnol Biochem. 2004. 68(2):352–359.
22. Lowe ME. Molecular mechanisms of rat and human pancreatic triglyceride lipases. J Nutr. 1997. 127(4):549–557.
23. Lowe ME. The triglyceride lipases of the pancreas. J Lipid Res. 2002. 43(12):2007–2016.
24. Artenie R, Ungureanu D, Artenie A, Botnariu G, Anisie E. [HDL-cholesterol--active or passive participant in the pathogenesis of atherosclerosis]. Rev Med Chir Soc Med Nat Iasi. 2003. 107(2):282–287.
25. Sancho-Rodriguez N, Aviles-Plaza FV, Granero-Fernandez E, Hernandez-Martinez AM, Albaladejo-Oton MD, Martinez-Mernandez P, Parra-Pallares S. Observational study of lipid profile and LDL particle size in patients with metabolic syndrome. Lipids Health Dis. 2011. 10:162.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download