Abstract
In this study, we evaluate a method for the early diagnosis of radiodermatitis for use in the prevention and therapy of this condition. Hairless mice (SKH1-hr) were used to study the early diagnosis of radiodermatitis. Lactate dehydrogenase (LDH, EC 1.1.1.27) isozymes were analyzed using native-polyacrylamide gel electrophoresis and western blotting of blood serum and tissues collected from SKH1-hr mice. Radiodermatitis developed 24 days after the first X-irradiation. Reduced spleen weight was observed after the last X-irradiation (P<0.05). Thereafter the weight increased until 24 days after the first irradiation, finally reaching levels comparable to those in the sham-irradiated control group. LDH activity was the highest in skeletal muscle and lowest in blood serum. LDH C4, A4, A3B, A2B2, AB3, and B4 isozymes were detected, in the mentioned order, from the cathode. This result was similar in other mouse strains. In the irradiated group, LDH A4 isozyme levels were reduced in the serum until inflammation occurred, whereas those of B4 isozyme were elevated. The subunits A and B followed a similar trend to that of LDH A4 and B4 isozyme, respectively. Importantly, antibodies against LDH B4 isozyme could prove useful in the early diagnosis of radiodermatitis.
Human radiodermatitis can be either acute or chronic [1,2]. Acute radiodermatitis is typically observed a few weeks after patients receive a high dose of ionizing radiation, and it manifests as erythema, epilation, desquamation, and erosion [3]. Because acute radiodermatitis is generally a side effect of radiotherapy used for cancer treatment, an understanding of its mechanism is important [4-6].
Skin damage can manifest differently depending on the kind of radiation, dose, dose rate, irradiated area, age, and health condition of the patient [3]. Free radicals are produced by irradiation, and they have an effect on the expression of various genes related to the cell cycle, which can result in delayed cell cycle, apoptosis, and tumorigenesis [7]. Previous research has examined the gene expressions of radiodermatitis-related cytokines after irradiation [8]; however, early diagnosis of radiodermatitis has been difficult owing to the complexity of result interpretation. In addition, research on radiodermatitis diagnosis is insufficient because cancer therapy is highly important, and little can be done to alleviate the side effects.
Lactate dehydrogenase (LDH, EC 1.1.1.27) in vertebrates catalyzes the interconversion of lactate and pyruvate [9]. It exists as a tetrameric isozyme containing A, B, and C subunits [10]. The LDH A4 isozyme (which primarily acts as a pyruvate reductase) has been predominantly detected in anaerobic tissues, while the B4 isozyme (which primarily acts as a lactate oxidase) has been detected predominantly in aerobic tissues [11-13]. However, the function of mammalian testis-specific LDH C4 isozyme has not been hitherto clarified [14].
LDH has been demonstrated to function as a marker for identifying cell damage in body fluids [15]. Because the expression patterns of the LDH isozymes are different for aerobic and anaerobic metabolic pathways, they can be used for the diagnosis of many different diseases. For example, the LDH A4 isozyme increases during viral hepatitis [16] and gastric cancer [17], while the B4 isozyme increases during acute myocardial infarctions [18] and severe acute respiratory syndrome [19]. Interestingly, the testis-specific LDH C4 isozyme is expressed in cancer cells [20]. Although the expression patterns of the LDH isozymes after whole body irradiation have been previously studied [21], their expression patterns associated with radiodermatitis have not been well characterized.
Therefore, we examined the potential for the early diagnosis of radiation-induced dermatitis by performing biochemical and immunological detection of LDH isozymes. We hope our results will help in the prevention and therapy of radiodermatitis.
SKH1-hr mice were purchased from Orient Bio Inc. (Seongnam, Korea). The mice were 5 weeks old and weighed 20-23 g. Five to 6 mice were housed in each cage, kept under conventional conditions in specific pathogen-free facilities, and were adapted to a new environment for 5-6 days before the experiment. Four mice were allocated to test and control groups. These experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Laboratory Animal Research Center of Chungbuk National University (Approval Number CBNUA-240-1004-01).
Pyruvic acid, nicotinamide adenine dinucleotide (NAD+), reduced form of NAD (NADH), Coomassie brilliant blue G-250, bovine serum albumin (BSA), acrylamide, N,N'-methylene-bis-acrylamide, N,N,N',N'-tetramethylethylenediamine (TEMED), bromophenol blue (BPB), DL-lactic acid, nitro blue tetrazolium (NBT), phenazine methosulfate (PMS), and anti-rabbit IgG (peroxidase conjugate) were purchased from Sigma-Aldrich Co. (St. Louis, MO). The anti-LDH A4 isozyme [22] and anti-B4 isozyme [23] were obtained from the Animal Physiology Laboratory (Cheongju University, Korea).
Irradiation was limited to the dorso-posterior region to minimize the exposure of the internal organs. After taping the SKH1-hr mice to a 5 cm thick acrylic plate to maintain a backscatter, a linear accelerator (Mevatron 6700, Siemens, Germany) was used to X-irradiate the mice at a dose rate of 3 Gy/min, 12 Gy/day for four consecutive days. The irradiation dose was prescribed at the half-thickness of the buttock of each animal, which was equivalent to the Dmax (Depth of maximum dose, 1.5 cm) of a 6 megavoltage X-ray beam.
The irradiated SKH1-hr mice were sacrificed by cervical dislocation and 6 tissues - the skeletal muscle, heart, kidney, liver, spleen, and testis - were collected. Each tissue was ground in 3 or 7 volumes (v/w) 0.1 M potassium phosphate buffer (pH 6.85) by using Dounce and Potter-Elvehjem homogenizers in an ice-bath; next, the tissues were centrifuged in a refrigerated centrifuge at 20,000 g for 1 hr (20 PR-52D, Hitachi Koki Co. Ltd., Tokyo, Japan). The supernatants were used as extracts. Blood was collected by cardiac puncture [24], and serum was obtained after incubation at room temperature for 1 hr and at 4℃ for 6 hrs and centrifugation at 790 g for 20 min.
LDH activity assays were performed in 0.1M potassium phosphate buffer (pH 6.85) containing 1.50 mM pyruvate and 0.14 mM NADH. Changes in absorbency at 340 nm were measured using a spectrophotometer (UV-160A, Shimadzu Co. Ltd., Kyoto, Japan). One unit was defined as the amount of enzyme activity catalyzing the conversion of 1 µmole of substrate per min. Protein content of the extracts was determined according to the method described by Bradford [25] using BSA as the standard.
The protocol for native-PAGE, described by Davis [26], was followed using a Mighty Small II (SE 250, Hoefer Sci. Instr., San Francisco, CA) and a thermostatic circulator (EYELA CA-1100, Rikakikai Co. Ltd., Tokyo, Japan) to maintain samples at 4℃. Polyacrylamide gel plates containing 7.5%T and 2.67%C separation gel and 3%T and 2.67%C stacking gel were prepared. Samples with 25% sucrose and 0.025% BPB were electrophoresed on the gel plate at 100 V for 20 min and then at 200 V for 130 min in 5 mM Tris-glycine buffer (pH 8.3). LDH activity was determined by incubating the gel at 37℃ with a mixture of DL-lactic acid, NBT, PMS, and NAD+ and then fixing the gel in 15% acetic acid, as described by Whitt [27]. The relative activities of LDH isozymes were analyzed by BIO-1D++ software (Vilber Lourmat, Marne La Vallee, France).
Heart extracts from SKH1-hr mice were electrophoresed using native-PAGE and transferred to nitrocellulose membranes (Trans-Blot Transfer Medium, Bio-Rad Lab. Inc., Hercules, CA) at 25 V for 30 min by using the semi-dry transfer system (Trans-Blot SD cell, Bio-Rad Lab. Inc., Hercules, CA) in 15 mM Tris-glycine buffer (pH 8.3). Membranes were blocked with 5% skimmed milk in TBS (0.01 M Tris buffered saline, pH 7.5) for 1 hr and were then incubated with primary antibodies for 1 hr. LDH A4 and B4 isozymes were assayed using anti-LDH A4 isozyme [22] and anti-B4 isozyme [23]. After washing, the membranes were incubated with anti-rabbit IgG peroxidase-conjugated secondary antibodies for 1 hr and were then washed again. Immunodetection was performed using a chloronaphthol and hydrogen peroxide mixture.
In this experiment, we performed local X-irradiation of the dorso-posterior region to minimize organ damage. Radiodermatitis was induced in SKH1-hr mice without significant weight loss. Inflammation was observed 24 days after the first X-irradiation in the dorso-posterior region of SKH1-hr mice (Figure 1), according to which the radiodermatitis model was established.
Spleens were collected after X-irradiation to measure their weights. The spleen weights were significantly reduced immediately after the last irradiation (P<0.05). Thereafter, the weights increased until 24 days after the first irradiation (Figure 2).
LDH activities in the skeletal muscle, heart, kidney, liver, spleen, testis, and blood serum were 189.45, 59.81, 81.61, 120.00, 35.88, 16.67, and 0.57 units/mg, respectively (Table 1). Protein concentrations in skeletal muscle, heart, kidney, liver, spleen, testis, and blood serum were 42.18, 50.19, 86.38, 125.60, 127.02, 46.44, and 41.68 mg/g, respectively (Table 1). Therefore, it is apparent that protein concentrations in both the skeletal muscle and blood serum were similar. In addition, the specific activity of blood serum was very low.
Native-PAGE and western blot analysis were performed to detect the distribution of LDH isozymes in tissues of SKH1-hr mice. LDH C4, A4, A3B, A2B2, AB3, and B4 isozymes were detected, in the mentioned order, from the cathode (Figure 3). The LDH A4 isozyme was expressed predominantly in the skeletal muscle, liver, and spleen tissues, while the B4 isozyme was expressed predominantly in the testis (Figure 3).
Blood serum collection was the simplest form of tissue collection performed in this experiment. LDH activities in the serum were not affected by irradiation (P>0.05, data not shown). The LDH A4 isozyme was reduced on day 24 after the first irradiation, whereas the B4 isozyme increased on day 24 (Table 2), and the LDH C4 isozyme changed only slightly (Table 2). After irradiation, the production of LDH subunits A, B, and C were compared (Figure 4). Production of subunit A increased until day 7 after the first irradiation, after which it reduced until day 24. Subunit B showed a trend opposite to that of subunit A; in this aspect, they reflected the tendencies of their respective LDH isozymes. Production of subunit C remained unchanged (P>0.05, Figure 4).
Radiodermatitis was observed 24 days after the first X-irradiation. Although the total radiation dose was similar, this result was different from a previous study, which indicated that radiodermatitis was induced 18 days after the mice were first irradiated [28]. This difference is likely due to differences in the dose prescription point. We prescribed the radiation dose at the half-thickness of the buttock (1.5 cm), so the actual skin dose was far less than the prescription dose (48 Gy). Assuming that skin depth was 3 mm, the calculated skin dose was approximately 29 Gy.
Generally, spleen weight increased following radiodermatitis development [28]. The weights of the spleens reduced immediately after the last X-irradiation. Thereafter, the weights increased until the development of radiodermatitis. This result is likely due to reduction in the number of immune cells produced by the bone marrow [28,29].
The levels of LDH activity and protein concentration in the blood serum were examined. LDH activities in the skeletal muscle tissues were especially high; this has also been observed in hamsters, chickens, black-spotted pond frogs [23], javeline gobies [30], mandrin fish [22], catfish [31], and goby minnows [32]. LDH activities were very low in the blood serum, probably because LDH is primarily a cytoplasmic enzyme [23,33].
Isozymes can be identified if LDH isozyme expression patterns of each tissue are known and western blot analysis is performed [31]. Each LDH isozyme was identified by native-PAGE and western blot analysis. Our result is similar to the results of previous studies that used other mouse strains, indicating that there is no strain specificity [21,34]. Western blot analysis determined that some of the antigenic determinants of the LDH A4 isozyme in SKH1-hr mice and mandrin fish [22] were similar. An especially high immunoreactivity was observed between the B4 isozymes of SKH1-hr mice and Korean cattle [23], possibly because of a similar evolutionary relationship. The antigenic determinants of the testis-specific LDH C4 isozyme in SKH1-hr mice and an eye-specific LDH C4 isozyme in greenlings [35] were different due to tissue specificity (data not shown).
Notably, in our experiment, LDH B4 isozyme and subunit B were increased in the blood serum until inflammation occurred. It is likely that the production of LDH B4 isozyme and subunit B increased owing to the necessity of lactate oxidation before the development of radiodermatitis [11-13]. This study suggests that detection of antibodies against the LDH B4 isozymes may be useful for the early diagnosis of radiodermatitis. If an increase in LDH B4 isozyme is detected in the blood serum by the binding of the corresponding antibody, radiodermatitis may be potentially diagnosed at early stages.
Figures and Tables
Figure 1
Macrophotography of the radiodermatitis-induced SKH1-hr mice. The arrow indicates the region with inflammation.
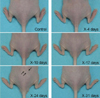
Figure 2
The weight of the spleen. C, Sham-irradiated control (○); X-irradiated (●). *Represents P<0.05 compared with the sham-irradiated control. Data are expressed as means±SD (n=4).

Figure 3
Native-polyacrylamide gel electrophoresis zymograms (a-g) and western blot analysis (h, i) of the lactate dehydrogenase (LDH) isozymes of SKH1-hr mice. Electrophoretic patterns of the skeletal muscle (a), heart (b), kidney (c), liver (d), spleen (e), testis (f), and blood serum (g). Western blotting of LDH A4 (h) and B4 isozyme (i). A4, LDH A4 isozyme; B4, LDH B4 isozyme; C4, LDH C4 isozyme; o, origin.
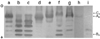
Figure 4
The relative expressions (%) of LDH subunit A (●), B (▼), and C (■) in the blood serum of the SKH1-hr mice with induced radiodermatitis. C, Sham-irradiated control. *Represents P<0.05 compared with the sham-irradiated control. Data are expressed as means±SD (n=4).

Acknowledgment
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (Ministry of Education, Science and Technology) [NRF-2007-359-C00033].
References
1. Cipollaro VA. Radiation dermatitis today. J Eur Acad Dermatol Venereol. 2001. 15(4):300–301.
2. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990. 57(4):751–773.
3. Malkinson FD, Keane JT. Radiobiology of the skin: review of some effects on epidermis and hair. J Invest Dermatol. 1981. 77(1):133–138.
4. Bernstein EF, Sullivan FJ, Mitchell JB, Salomon GD, Glatstein E. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg. 1993. 20(3):435–453.
5. Ertekin MV, Tekin SB, Erdogan F, Karslioglu I, Gepdiremen A, Sezen O, Balci E, Gündogdu C. The effect of zinc sulphate in the prevention of radiation-induced dermatitis. J Radiat Res. 2004. 45(4):543–548.
6. Murakami R, Baba Y, Nishimura R, Furusawa M, Yokoyama T, Yamashita Y, Takahashi M, Yamashita N, Ono T. The effect of azelastine on acute radiation dermatitis in mice models. Int J Radiat Oncol Biol Phys. 1997. 37(4):907–911.
7. Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998. 5(6):401–414.
8. Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000. 47(2):277–290.
9. Holbrook JJ, Liljas A, Steindel SJ, Rossmann MG. Boyer PD, editor. Lactate dehydrogenase. The Enzymes. 1975. Vol. 11:3rd ed. New York: Academic Press Inc;191–292.
10. Markert CL, Shaklee JB, Whitt GS. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science. 1975. 189(4197):102–114.
11. Everse J, Kaplan NO. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973. 37:61–133.
12. Hochachka PW. 1980. Living without Oxygen. 1980. Cambridge: Harvard University Press;181.
13. Val AL, Almeida-Val VMF. Fishes of the Amazon and Their Environment. 1995. Berlin: Springer-Verlag;55–56.
14. Goldberg E, Eddy EM, Duan C, Odet F. LDHC: the ultimate testis-specific gene. J Androl. 2010. 31(1):86–94.
15. Sevinc A, Sari R, Fadillioglu E. The utility of lactate dehydrogenase isoenzyme pattern in the diagnostic evaluation of malignant and nonmalignant ascites. J Natl Med Assoc. 2005. 97(1):79–84.
16. Andriutsa KA, Andriutsa AK. Diagnostic and pathogenetic significance of determining lactate dehydrogenase isoenzyme activity in various biological media in patients with viral hepatitis. Ter Arkh. 1987. 59(2):89–94.
17. Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008. 15(8):2336–2344.
18. Painter PC, Van Meter S, Dabbs RL, Clement GE. Analytical evaluation and comparison of Dupont aca lactate dehydrogenase-1 (LD1) isoenzyme assay diagnostic efficiency for acute myocardial infarction detection with other LD1 methods and aca CK-MB. A two-site study. Angiology. 1994. 45(7):585–595.
19. Lam CW, Chan MH, Wong CK. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev. 2004. 25(2):121–132.
20. Koslowski M, Türeci O, Bell C, Krause P, Lehr HA, Brunner J, Seitz G, Nestle FO, Huber C, Sahin U. Multiple splice variants of lactate dehydrogenase C selectively expressed in human cancer. Cancer Res. 2002. 62(22):6750–6755.
21. Park HD, Yum JJ. Redistribution of lactate dehydrogenase isozymes and morphology of tissues in Mus musculus after irradiation. Korean J Environ Biol. 1999. 17(3):263–270.
22. Cho SK, Ku B, An H, Park EM, Park SY, Kim JB, Yum JJ. Purification and characterization of lactate dehydrogenase A4 isozyme in mandrin fish (Siniperca scherzeri). J Life Sci. 2009. 19(2):256–263.
23. Cho SK. Mitochondrial lactate dehydrogenase in tissues ofvertebrate. 2000. Korea: Cheongju University;88. Ph.D. Thesis.
24. Donovan J, Brown P. Coligan JE, editor. Cardiac puncture of rabbit. Current Protocols in Immunology. 1991. Vol. 1. New York: John Wiley & Sons Inc;Unit 1.7.6.
25. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.
26. Davis BJ. Disc electrophoresis. II. Method and application to human serum proteins. Ann N Y Acad Sci. 1964. 121:404–427.
27. Whitt GS. Developmental genetics of the lactate dehydrogenase isozymes of fish. J Exp Zool. 1970. 175(1):1–35.
28. Maeng HG, Kim DN, Cho SK, Cha JH, Kim TY, Lee YS, Choi DK, Lee JH, Cho MJ, Kwon HJ, Lee SK. Altered immune cell proportions in the radiodermatitis induced hairless mice-1 (HR-1). J Radiat Res. 2006. 47(1):9–17.
29. Oluwole SF, Engelstad K, De Rosa C, Wang TS, Fawwaz RA, Reemtsma K, Hardy MA. Migration patterns of dendritic cells in the rat: comparison of the effects of gamma and UV-B irradiation on the migration of dendritic cells and Lymphocytes. Cell Immunol. 1991. 133(2):390–407.
30. Yum JJ. Characterization of lactate dehydrogenase in Acanthogobius hasta. J Life Sci. 2008. 18(2):264–272.
31. Cho SK, Yum JJ. Lactate dehydrogenase isozyme of hypoxia tropical catfish (Pangasius polyuranodon, Hypostomus plecostomus). J Life Sci. 2004. 14(4):702–707.
32. Kim MO, Yum JJ. Purification, kinetics and immunochemistry of two homotetrameric lactate dehydrogenase isozymes in Pseudogobio esocinus (Cypriniformes). Korean J Zool. 1989. 32(4):420–428.
33. Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics. 2011. 43(5):255–264.
34. Li SS, O'Brien DA, Hou EW, Versola J, Rockett DL, Eddy EM. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol Reprod. 1989. 40(1):173–180.
35. Cho SK, Yum JJ. Purification and characterization of eye-specific lactate dehydrogenase C4 isozyme in greenling (Hexagrammos otakii). J Life Sci. 2011. 21(11):1565–1572.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download