Abstract
This study used a biomechanical test to evaluate the effects of pentoxifylline administration on the wound healing process of an experimental pressure sore induced in rats. Under general anesthesia and sterile conditions, experimental pressure sores generated by no. 25 Halsted mosquito forceps were inflicted on 12 adult male rats. Pentoxifylline was injected intraperitoneally at a dose of 50 mg/kg daily from the day the pressure sore was generated, for a period of 20 days. At the end of 20 days, rats were sacrificed and skin samples extracted. Samples were biomechanically examined by a material testing instrument for maximum stress (N mm2), work up to maximum force (N), and elastic stiffness (N/mm). In the experimental group, maximum stress (2.05±0.15) and work up to maximum force (N/mm) (63.75±4.97) were significantly higher than the control group (1.3±0.27 and 43.3±14.96, P=0.002 and P=0.035, respectively). Pentoxifylline administration significantly accelerated the wound healing process in experimental rats with pressure sores, compared to that of the control group.
Pressure sores are a major health problem currently affecting approximately three million adults [1-4].
Mostly, pressure sores occur in patients who are immobile or unable to change their body positions. In this condition the patient's dermal tissues are at increased risk for necrosis of the skin, subcutaneous tissues, and muscles.
Pressure sores are defined as areas of skin discoloration or damage which persist following the removal of pressure and which are likely to have resulted from the effects of pressure on the tissues. Throughout history, pressure sores have previously been called decubitus ulcers, bed sores. Scientists have attempted to use these terms in order to identify and describe the pathophysiology of wounds that result from physical stress. However, bed sore could not justify the existence of a pressure sore that occurs for any other reason, except in bed such as those pressure sores that occur in individuals who use wheelchairs. Today, pressure sore is the best description for this type of sore, as these lesions are multifactorial in nature and may occur anywhere on the body [1].
Stress, time, spasticity, infection, edema, nerve transaction, and poor nutrition are considered the main factors which lead to pressure sores or at least have a role in their development. Approximately more than 60% of pressure sores occur in hospitalized patients and these numbers are increasing. This is possibly due to the increased numbers of elderly people who need to be hospitalized, because aging causes a reduced performance in one third to half of them. Health care providers and hospital administrators are legally obligated to prevent patients from acquiring pressure sores and physical weaknesses. In order to achieve this goal and prevent the occurrence of pressure sores, intensive preventative measures are warranted [1]. As mentioned earlier, the primary cause of pressure sores is based on the exerted pressure and permanent forces on the patient's dermal tissues, whereby the supply of oxygen is reduced or cutoff causing tissue necrosis [2,3].
Pressure sores remain a major challenge in the medical world [4,5]. In English hospitals, the prevalence of pressure sores has ranged from 9.6 to 11.9% among adult patients; in those who had surgery the range was 12%, whereas it was 22% in elderly patients. Statistics taken from the English population showed that the prevalence range in adults was 4.4% and in children it was 6.8%. Studies in the United States and Canada have shown that the prevalence range and incidence range vary according to environmental conditions. For example, in acute care settings, there was a prevalence of 4.7% to 29.7% [5]. A similar study in European hospitals showed an 18.1% prevalence of pressure sores [6]. According to reports by the Consultation Secretariat of the National Association of Pressure Ulcers, a prevalence range of 10% to 18% was seen in general acute care units [7].
Pentoxifylline is a xanthine derivative and like other methylated xanthine derivatives, it is a competitive nonselective phosphodiesterase inhibitor [8] that raises intracellular cAMP, activates PKA, inhibits TNF-alpha [9,10] and leukotriene [11] synthesis, and reduces inflammation and innate immunity [11]. In addition, pentoxifylline improves red blood cell deformability, reduces blood viscosity and decreases the potential for platelet aggregation and thrombus formation [12].
Pentoxifylline improves blood flow through peripheral blood vessels and therefore assists with blood circulation in the arms and legs (e.g., intermittent claudication), and the brain in cases of vascular dementia.
A large volume of studies have identified the positive effects of pentoxifylline administration on skin flaps [13-15], venous ulcers, skin ulcers in both healthy [16-19] and diabetic mice [20], colitis, stomach ulcers, and small and large bowel anastomosis in experimental ischemic conditions [21-24].
However, in most studies clinical observations have been used as criteria to evaluate the effects of pentoxifylline on its ability to heal sores in patients. Unfortunately, quantitative assessment criteria, such as the effects of pentoxifylline on biomechanical factors in healing skin sores have been less considered. Factors such as metabolic, circulatory, and neurotrophic changes, in addition to complications arising from ischemia are additional factors that create pressure sores [25]. It is known that ischemia and reperfusion are the most important factors in the pathogenesis of pressure sore development [26]. Recent studies have shown the positive effects of pentoxifylline in cases of ischemic conditions [15,16,20,21]. In a review of the literature, despite numerous reviews that have discussed and identified the influence of pentoxifylline on wound healing, no study has investigated the influence of pentoxifylline on pressure sores. Thus the present study has been designed to investigate the effects of pentoxifylline administration on healing experimental pressure sores in rats by using a biomechanical evaluating method.
Twenty adult male Wistar rats with a mean age of twelve weeks (10-14 weeks) and mean body weight of 250 grams (230-270 grams) were purchased from the Pasteur Institute of Iran. During the study, rats were maintained in individual cages in an animal house with a light-dark cycle (12 h light, 12 h dark) and access to water and food ad libitum. Animals were kept for at least two weeks in the animal house until they acclimated to their surroundings. All study procedures were approved by the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
In a pilot study, four degrees of pressure (low, medium, high and very high) were inflicted by using nos. 25 and 13 Halsted mosquito forceps on eight rats (two rats per degree of pressure). The rats' skins were held between two clamps of forceps for 2 hours, after which pressure was released for about 30 min (Figure 1). As with the main experiment, this procedure was repeated 12 times during three consecutive days for each grade.
Histological studies on the samples which were taken at the end of the seventh day showed that pressure sores were created only in the skins that received very high pressure with the no. 25 forceps (Figure 2).
In the main study, twelve additional rats were anesthetized by intramuscular injections on day 0, the hair from the dorsal regions were shaved and cleaned with 70% alcohol and povidine iodine. Then, under sterile conditions, the skin was raised and double-folded from the middle region. Approximately a one centimeter length of skin was held under the highest pressure grade of the no.25 Halsted mosquito forceps for 2 h (Figure 1) after which their skins were released for about 30 min to induce an experimental model of ischemia and perfusion on the skin tissue [27]. A thin sheet of aluminum (dimensions, 3×5 mm) was laid between contact sides of the skin and forceps clamps to enable equal distribution of the pressure on all parts of the skin. Ischemia (2 h) and perfusion (30 min) were applied for a period of 12 times (four periods per day) during 3 consecutive days. During those courses, the rats were anesthetized by injection of anesthetic drugs. From the beginning of the third day, rats were followed for seven days until the presence of pressure sore models were noted on their skins. At the end of the seventh day, we randomly divided the twelve rats into two groups, control and experimental. The experimental group received intraperitoneal injections of 50 mg/kg pentoxifylline [28]. Control groups received a similar volume of saline. These injections were administered daily for 20 consecutive days.
Rats were sacrificed by inhalation of chloroform at the end of day 20 and skin samples taken (5 cm of length×5 mm of breadth) from the wound and surrounding skin area, with wounds located in the center of the samples. Samples were placed in a piece of gas packing wound impregnated with saline solution and maintained at -20℃. Prior to conducting the tensiometry test, samples were slowly thawed at room temperature. We used a material testing instrument (Zwick/Roell Z 2-5-PH1F, Germany) for tensiometry. Each ends of the samples were fixed in both movable and stable clamps of the device. Data on the thickness and width of the sample and speed of the moving clamp were transferred to a computer connected to the material testing instrument. The mobile clamps were vertically moved away from the stable clamp at a rate of 15 mm per min. The computer recorded the curve of load-deformation for each sample.
The maximum force, which disrupted microscopically the sample from wound site was calculated from the curve. Maximum force value was divided on the cross section (breadth×depth ) of the sample. The maximum stress based on (N/mm2) was derived from that. The elastic stiffness of the samples, the maximum gradient of the linear curve based on (N/mm; the curve in the elastic phase) and work up to the maximum force (N/mm; absorption of energy) were calculated by the computer (Figure 3) [29].
Data were analyzed and compared by the independent sample student's t-test. P<0.05 was considered significant. Data were presented as mean±SD. Figures 4-6 show the results of this study. All rats survived, with no secretion or symptoms of wound infection in both groups during the investigation.
Statistical analyses showed a significant difference between the maximum stress and work up to maximum force in the experimental group. Work up to maximum force in the experimental group was 43.3±14.96, and in the control group it was 63.75±4.97 (P=0.035; Figure 4). The mean±SD of the maximum stress in the control group was 2.05±0.154 and in the experimental group, it was 1.3±0.278 (P=0.002; Figure 5). Elastic stiffness in the control group was 5.68±1.19 and in the experimental group, it was 7.69±1.61 (P=0.058; Figure 6).
Administration of pentoxifylline increased the parameters of the biomechanical tests, such as maximal stress and work up to maximum force, compared with the control group.
According to the results of this study, administration of pentoxifylline accelerated the healing process in pressure sores, as confirmed by the results obtained through evaluation of the biomechanical factors.
The biomechanical findings showed that the tensile strength of the pressure sores was higher in the experimental group treated by pentoxifylline, compared with the control group, which was shown by the increased maximum stress in the experimental group.
The work up to maximum force by pressure sores was higher in the rats treated by pentoxifylline compared to the control groups, which showed a higher rate of healing and regeneration of tissue repair in the experimental group.
In the experimental group the elastic stiffness of the pressure sores approximated significance (P=0.058), which indicated that administration of pentoxifylline gave more elasticity to repaired tissues when compared with the control group [29]. There was no statistically significant difference between groups in work up to the maximum force, however the increased maximum stress and elastic stiffness were important in repaired samples in the experimental group, in which this showed that the re-opening of sores occurred less in this group [29].
According to literature reviews, a large volume of studies have demonstrated that pentoxifylline has a positive effect on skin flap survival [13-15], venous sores [16-19], wounds with full thicknesses of skin in healthy and diabetic mice [20], experimental colitis, stomach ulcers, and intestinal ischemia [21-24]. The current study, for the first time, has reported the effects of pentoxifylline administration on an experimental model of pressure sores.
The present study involved only biomechanical tests and the effects of pentoxifylline on the sore healing process. Our results have suggested that administration of pentoxifylline positively effected strength and rate of maturity in tissue repair. Similar results have been obtained in research by Karasoy et al. and Tireli et al. on skin sores and small intestine [20,23]. Karasoy et al. have observed that the administration of pentoxifylline significantly increased tensile strength of skin wounds in healthy mice compared to healthy controls [20]. However they did not find any significant difference in histological studies between the groups. Their justification for increasing the tensile strength of wounds treated by pentoxifylline was that tissue perfusion increased in these wounds [20].
Tireli and his colleagues have shown that administration of pentoxifylline during the healing process of intestinal grafts, lead to increase in tensile strength and the amount of proline amino acid in ischemia and reperfusion wounds [23].
The present study and studies by Karasoy et al. [20] and Tireli et al. [23] have shown that wound tensile strength in the experimental groups significantly increased. This indicates that fibroblasts are more active in collagen synthesis. However findings by Isacc et al., Dans and Isseroff, differ due to the use of a culture medium condition [30,31].
Isacc et al. investigated the effects of pentoxifylline on human fibroblasts derived from hypertrophied scars after burns, where they reported that these cells synthesized less type III collagen fibers [30].
Dans and Isserof investigated the combined effects of pentoxifylline and interferon on fibroblasts and a model of wound contraction in vitro. They have concluded that this material might delay wound contractions in vivo and decrease production of scar tissue in sores associated with severe scar tissue [31]. However, it seems that more cellular and molecular research must be performed in this field to clarify the ambiguities of those researches.
Review of the literature has shown that a large volume of studies on the use of pentoxifylline in experimental models of skin flaps have been undertaken [13-15]. In those studies pentoxifylline was injected in the animals, surgery was then performed, skin flaps were created, followed by administration of pentoxifylline for a period of time.
In one such study [14], the administration of pentoxifylline began seven days before surgery in the first stage, but did not lead to a significant increase in flap survival. Thus in the second group, pentoxifylline was given 14 days before flap surgery, where a significant increase in skin flap survival was observed. This finding showed that administration of pentoxifylline prior to surgery was necessary to achieve positive results. However, in that research the flap model was used under ischemia and reperfusion conditions, which varied from pressure sores. In the present study, pentoxifylline was administered after induction of pressure sores, where afterwards, a positive effect was observed and reported.
In all three studies [13-15], the dosage of pentoxifylline was 20 mg/kg, which was lower than the dose used in the present study. It was likely, if more pentoxifylline were used in that research, that a positive effect on flap survival could be seen without any preoperative administration of pentoxifylline.
The scientists, according to a review article by Bath and Bath-Hextall, included the application of pentoxifylline, propentofylline, and pentofilin on acute ischemic stroke. Based on the results of clinical trial research, they concluded that pentoxifylline may reduce death after acute ischemic stroke, however, unfortunately the number of studies and the number of patients was low. In these studies, the research design was not ideal. Therefore, it seems that more research is necessary. The researchers suggested that pentoxifylline should not be commonly used in acute ischemic stroke [32].
According to one recent study that has evaluated the effect of pentoxifylline on skin flap survival, it was shown that the ultrastructure of the endothelium in the pentoxifylline administered group, compared with the control group showed that although chromatin structure in the endothelium of control groups was intact, the mitochondria were swollen and their crown were disruptive. Large pinocytotic vesicles were frequently observed in the cytoplasm of these cells, which seemed to be derived from the smooth endoplasmic reticulum network. In that study Bayat et al. reported that in the pentoxifylline-treated group the cell membranes were thicker than normal, but the chromatin structure in the endothelium of blood vessels was normal. The cell and nuclear membrane had two layers and mitochondria and pinocytotic vesicles were normal [15].
Due to the major role of mitochondria in health and cell activity, and considering that the flap model investigated in that study was in an ischemic condition [15], we may conclude that in the present study the positive effects of pentoxifylline on endothelial cells and on acceleration of the process of healing pressure sores in rats could be considered an important point for future studies.
In conclusion, we have shown that the wound healing process in an experimental pressure sore model in rats was accelerated by administration of 50 mg/kg of pentoxifylline compared with the control group as was observed by biomechanical testing of the repaired tissue.
Additional research is needed in this field using histological, biochemical, and molecular techniques.
Figures and Tables
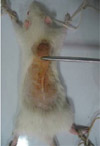 | Figure 1Creation of the experimental pressure sore using no. 20 Halsted mosquito forceps in the rat's skin. |
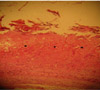 | Figure 2Histological view of an experimental pressure sore caused by maximum pressure generated with no. 20 Halsted mosquito forceps. Location of the wound is noted by stars. Hematoxylin and eosin stain. |
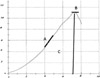 | Figure 3Curve of the stress-strain obtained by the tensiometry test in experimental and control groups, A: Elastic stiffness, B: Maximum force, C: Work up to maximum force. Magnification 10×10. |
 | Figure 4Mean±SD of work up to maximum force in the control and experimental groups, compared by student's t-test. Statistical analysis showed significant differences between the studied groups (P=0.035). |
Acknowledgments
We wish to thank the Vice Chancellor of Research at Shahid Beheshti University of Medical Sciences, Tehran, Iran for financial support.
References
1. Bass MJ, Phillips LG. Pressure sores. Curr Probl Surg. 2007. 44:101–143.
2. Defloor T. The risk of pressure sores: a conceptual scheme. J Clin Nurs. 1999. 8(2):206–216.
3. Meijer JH, Germs PH, Schneider H, Ribbe MW. Susceptibility to decubitus ulcer formation. Arch Phys Med Rehabil. 1994. 75(3):318–323.
4. Papanikolaou P, Lyne P, Anthony D. Risk assessment scales for pressure ulcers: a methodological review. Int J Nurs Stud. 2007. 44(2):285–296.
5. Shahin ES, Dassen T, Halfens RJ. Pressure ulcer prevalence and incidence in intensive care patients: a literature review. Nurs Crit Care. 2008. 13(2):71–79.
6. Clark M, Bours G, Delfoor T. Summery report on the prevalence of pressure ulcer. EPUAP Rev. 2002. 4:49–57.
7. NPUAP. Pressure ulcer in America; prevalence, incidence and implications for the future. 2001. Reston VA: NPUAP.
8. Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol. 2001. 108(5):671–680.
9. Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R. Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition. Clinics (Sao Paulo). 2008. 63(3):321–328.
10. Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. Am J Respir Crit Care Med. 1999. 159(2):508–511.
11. Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol. 2005. 174(2):589–594.
12. Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987. 34(1):50–97.
13. Yessenow RS, Maves MD. The effects of pentoxifylline on random skin flap survival. Arch Otolaryngol Head Neck Surg. 1989. 115(2):179–181.
14. Pratt MF. Edmund Prince Fowler Award Thesis. Evaluation of random skin flap survival in a porcine model. Laryngoscope. 1996. 106(6):700–712.
15. Bayat M, Chelcheraghi F, Piryaei A, Rakhshan M, Mohseniefar Z, Rezaie F, Bayat M, Shemshadi H, Sadeghi Y. The effect of 30-day pretreatment with pentoxifylline on the survival of a random skin flap in the rat: an ultrastructural and biomechanical evaluation. Med Sci Monit. 2006. 12(6):BR201–BR207.
16. Falanga V, Fujitani RM, Diaz C, Hunter G, Jorizzo J, Lawrence PF, Lee BY, Menzoian JO, Tretbar LL, Holloway GA, Hoballah J, Seabrook GR, McMillan DE, Wolf W. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo-controlled trial. Wound Repair Regen. 1999. 7(4):208–213.
17. Dale JJ, Ruckley CV, Harper DR, Gibson B, Nelson EA, Prescott RJ. Randomised, double blind placebo controlled trial of pentoxifylline in the treatment of venous leg ulcers. BMJ. 1999. 319(7214):875–878.
18. Jull A, Waters J, Arroll B. Pentoxifylline for treatment of venous leg ulcers: a systematic review. Lancet. 2002. 359(9317):1550–1554.
19. De Sanctis MT, Belcaro G, Cesarone MR, Ippolito E, Nicolaides AN, Incandela L, Geroulakos G. Treatment of venous ulcers with pentoxifylline: a 12-month, double-blind, placebo controlled trial. Microcirculation and healing. Angiology. 2002. 53:S49–S51.
20. Karasoy A, Kuran I, Turan T, Hacikerim S, Bas L, Sungan A. The effect of pentoxifylline on the healing of full-thickness skin defect on diabetic and normal rat. Eur J Plast Surg. 2002. 25:253–257.
21. Peterson TC, Davey K. Effect of acute pentoxifylline treatment in an experimental model of colitis. Aliment Pharmacol Ther. 1997. 11(3):575–580.
22. Shimizu N, Watanabe T, Arakawa T, Fujiwara Y, Higuchi K, Kuroki T. Pentoxifylline accelerates gastric ulcer healing in rats: roles of tumor necrosis factor alpha and neutrophils during the early phase of ulcer healing. Digestion. 2000. 61:157–164.
23. Tireli GA, Salman T, Ozbey H, Abbasoglu L, Toker G, Celik A. The effect of pentoxifylline on intestinal anastomotic healing after ischemia. Pediatr Surg Int. 2003. 19(1-2):88–90.
24. Parra-Membrives P, Ruiz-Luque V, Escudero-Severín C, Aguilar-Luque J, Méndez-García V. Effect of pentoxifylline on the healing of ischemic colorectal anastomoses. Dis Colon Rectum. 2007. 50(3):369–375.
25. Kosiak M. Etiology and pathology of ischemic ulcers. Arch Phys Med Rehabil. 1959. 40(2):62–69.
26. Werns SW, Lucchesi BR. Free radicals and ischemic tissue injury. Trends Pharmacol Sci. 1990. 11(4):161–166.
27. Tsutakawa S, Kobayashi D, Kusama M, Moriya T, Nakahata N. Nicotine enhances skin necrosis and expression of inflammatory mediators in a rat pressure ulcer model. Br J Dermatol. 2009. 161(5):1020–1027.
28. Reddy GK, Stehno-Bittel L, Enwemeka CS. Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen. 2001. 9(3):248–255.
29. Bannister LH. Williams PH, Bannister LH, Berry MM, editors. Integumental system: Skin and Breast. Gray's Anatomy. 1995. 38th ed. Edinburgh: Churchill Livingstone;375–424.
30. Isaac C, Mathor MB, Bariani G, Paggiaro AO, Herson MR, Goldenstein-Schainberg C, Carrasco S, Teodoro WR, Yoshinari NH, Ferreira MC. Pentoxifylline modifies three-dimensional collagen lattice model contraction and expression of collagen types I and III by human fibroblasts derived from post-burn hypertrophic scars and from normal skin. Burns. 2009. 35(5):701–706.
31. Dans MJ, Isseroff R. Inhibition of collagen lattice contraction by pentoxifylline and interferon-alpha, -beta, and -gamma. J Invest Dermatol. 1994. 102(1):118–121.
32. Bath PM, Bath-Hextall FJ. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database Syst Rev. 2004. 3:CD000162.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download