Abstract
The purpose of this study was to investigate the anti-hyperlipidemic effect of soy bean extract solution fermented by Bacillus subtilis MORI (BTD-1E) in obese db/db mice. Eight-week-old male db/db mice were administered 33.3 mg/kg BTD-1E solution orally once a day for four weeks. The BTD-1E group showed significantly lower body weight compared with the db control group (P<0.05). The BTD-1E group showed significantly lower serum total cholesterol and LDL cholesterol levels compared with the db control group, respectively (P<0.05, P<0.01). The BTD-1E group showed significantly decreased liver weight relative to final body weight compared with the db control group (P<0.01). After four weeks of BTD-1E administration, lipid droplets in the liver were apparently decreased in the BTD-1E group compared to the db control group. In summary, our results suggest that BTD-1E has an anti-hyperlipidemic effect in the obese mouse model.
For the past decades, obesity has become a serious worldwide health problem largely due to high caloric intake and insufficient exercise [1]. Physiologically obesity is a disorder of energy balance and is fundamentally considered as a disorder of lipid metabolism [2]. It has been reported that obesity has a large effect on lipoprotein metabolism, leading to elevated levels of low-density lipoprotein cholesterol (LDL-C) and total cholesterol (T-CHOL) [3,4]. Many health risks such as heart disease, type 2 diabetes mellitus (T2DM), stroke, hypertension, osteoarthritis, fatty liver disease, and some forms of cancer are observed to be associate with excess weight [5,6]. Life-insurance data and epidemiological studies confirm that increasing degrees of overweight and obesity are important predictors of mortality [6,7]. According to a recent report from the World Health Organization (WHO), it was estimated that 45% of Korean men and 54% of women were overweight in 2005, and the percentages are expected to increase to 66% and 67%, respectively by 2015 [8]. Although diet control and lifestyle changes remain the first steps in obesity management, lifestyle modification (alone) as a treatment for obesity is widely regarded as ineffective [9]. Thus, the use of anti-obesity drugs is potentially important as a supplement to lifestyle modification [10].
At present, several medications are available for obesity treatment: Orlistat, a gastric and pancreatic lipase inhibitor [11]; Sibutramine, a centrally acting monoamine-reuptake inhibitor [12]; Rimonabant, the first endocannabinoid (CB1)-receptor blocker [13]. Despite the anti-obesity effects, some critical adverse effects of these medications such as gastro-intestinal effects [14], increases in blood pressure and pulse rate [15], and increased incidence of mood-related disorder are reported [16].
1-deoxynojirimycin (DNJ) is known to be a strong intestinal α-glucosidase inhibitor (AGI) [17]. It has been reported that DNJ is a constituent part of mulberry leaves, dietary mulberry, and is also produced by several Bacillus and Streptomyces species isolated from soil [18,19]. DNJ potently inhibits α-glucosidase in the small intestine, binding to the active center of α-glucosidase [20]. It has been reported that DNJ reduces atherogenicity of LDL-C in patients with impaired glucose tolerance [21]. Cheik et al. have shown that soy product fermented by Enterococcus faecium and Lactobacillus Jugurti significantly decreases the lipogenesis rate and increases in lipolysis rate in tissue adipocytes and liver in rats with hypercholestrolemic diet [22]. The preventive effect of DNJ and AGI on T2DM has been extensively studied [23-24].
Recently, a paper has reported that a new species isolated from Bacillus subtilis MORI produces both AGI and DNJ in high quantities, and is present in traditionally fermented Korean soybean [25]. The DNJ content and AGI activity of Bacillus subtilis MORI-fermented soybean extract (BTD-1) were approximately 5-fold higher than those of mulberry leaves [26,27]. Our previous study showed the glucose-lowering effect of BTD-1 in db/db mice. However, the hypolipidemic effect of BTD-1 has not been completely studied.
The present study was thus designed to investigate the lipid-lowering effect of water extract of BTD-1 (BTD-1E) in db/db mice.
Twelve male db/db mice (five weeks old, Central Lab. Animal, Inc., Seoul, Korea) were used after a three weeks acclimatization period. The mice were divided into two groups of six. The db control group was administered with 0.9% saline only, whereas the BTD-1E group was administered with 33.3 mg/kg of BTD-1E solution orally once a day for four weeks. The animals were maintained in the experimental animal center at Hallym University, Korea. The facility was maintained at 22±2℃, in 55±10% relative humidity, and an artificial 12 h light and 12 h dark cycle was maintained. A normal rodent pellet diet (Jeiljedang, Seoul, Korea) and water were supplied ad libitum. This animal study was conducted in accordance with the guidelines and the approval of the Institutional Animal Care and Use Committee of Hallym University (Hallym-2010-74).
BTD-1E was supplied by Biotopia Co. (Chuncheon, Korea). The 5% (w/v) defatted soybean meal was fermented for five days at 37℃ by Bacillus subtilis MORI (KCCM-10450P), which had been isolated from chungkookjang, a Korean traditional food. BTD-1 powder was made into a spray by drying the culture supernatant and the excipient so that they were 1:1 (v/v). Twenty grams of BTD-1 powder was well mixed with 100 mL distilled water. The mixture was extracted for 1 hr at 60℃ in a waterbath. Then the reactant was centrifuged at 10,000 g for 10 min. The supernatant of BTD-1E was used in this animal study. The DNJ component of BTD-1 powder is 0.944±0.04% (w/w). The α-glucosidase inhibitor unit of BTD-1 was 593,200 GIU/g and the 50% inhibitory concentration (IC50) was 1.7 µg/mL. The dosage of the active ingredients in BTD-1E was considered with the differences between human (60 kg adult) and mouse metabolic in mind. The dosage of oral administration of BTD-1E was 2,000 GIU/kg (mouse body weight) of GIU and the DNJ concentration was 0.37 mg/kg.
Throughout the four week experimentation period, body weight and food intake were measured weekly.
Blood was collected from the orbital plexus after an overnight fast (15 h), weekly. We measured T-CHOL, LDL-C, HDL cholesterol (HDL-C), and triglyceride (TG). For these measurements we used a biochemical analyzer (Kornelab20XT, Thermo, Espoo, Finland) with commercial reagents from Thermo Fisher Scientific (Espoo, Finland).
Organ weights, including that of the liver, kidney, testis, spleen, and epiderminal fats (EPDF) were carefully separated and weighed. The relative organ weight (%) was calculated based on final body weight.
At the end of the experiment, livers were collected from mice under anesthesia with diethyl ether after 15 h of fasting. The livers were fixed in 4% paraformaldehyde for 24 h. The fixed livers were then transferred to a 20% sucrose solution for 48 h. Then the livers were treated with OCT compound for 24 h. The tissues were processed routinely for frozen-sectioned 10 µm in thickness and stained with Oil Red O solution (O-0625, SIGMA-ALDRICH, St. Louis, USA). For nucleus staining, the tissues were stained with hematoxylin and eosin according to general procedures. Morphology of the livers was examined under an inverted microscope (ZEISS, Oberkochen, Germany) and the images were captured using the Axiovision Rel. 4.7 program.
Body weight and food consumption were measured once a week during the experimental period. BTD-1E treatment significantly decreased mean body weight in the mice throughout the experimental period compared with that of db control group (P<0.05) (Figure 1A). Average food consumption was slightly higher in the BTD-1E group compared with the db control group (Figure 1B). The difference in the food consumption of the mice treated with BTD-1E was not significant when compared with that of the db control group.
The contents of various types of lipid in serum including T-CHOL, LDL-C, HDL-C, and TG were analyzed in the db/db mice treated with the BTD-1E solution. At 3 and 4 weeks, the BTD-1E group showed significantly lower T-CHOL levels: 74.6 and 64.8% of that of the db control group, respectively (P<0.05) (Figure 2A). At 2, 3, and 4 weeks, the BTD-1E group showed significantly lower LDL-C levels: 63.4, 39.4, and 64.8% of the db control group, respectively (P<0.05, P<0.01, P<0.01) (Figure 2B). The HDL-C level was slightly lower in the BTD-1E group compared with the db control group (Figure 2C). TG level was slightly higher in the BTD-1E group compared with the db control group (Figure 2D). The differences in the HDL-C and TG levels of the mice treated with BTD-1E were not significant when compared with the db control group.
Changes of liver, spleen, testis, kidney, and EPDF weights were measured in relation to final body weight (Figure 3). The relative final body weights and liver weights of the BTD-1E group were only 88.1 and 79.7% of those of the db control group, respectively (P<0.05, P<0.01) (Figure 3A and 3B). The relative spleen, testis, and kidney weights of the BTD-1E group were 124, 105.7, and 128.2% of those of the db control group, respectively (P<0.01) (Figure 3C-3E). There were no significant differences in EPDF weight among experimental groups (Figure 3F). However, these results show the relative values to body weight, all actual weights of organs in BTD-1E group were lower than those of the db control group.
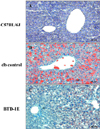
There were no lipid droplets in the livers of the C57BL/6J mice detected by Oil Red O stains (Figure 4A). Large fat droplets were present extensively in the livers of the db control mice (Figure 4B). The lipid droplets were remarkably less in the BTD-1E administered mice compared with the db control mice (Figure 4C).
We evaluated the inhibitory effect on lipid metabolism of feeding BTD-1E through the analysis of serum lipid contents and lipid accumulation in the liver in db/db mice for four weeks. The major findings of our studies are that BTD-1E decreases body weight due to its effects on lipid metabolism, as a dietary therapy for obesity. Overweight and obesity are usually defined using BMI [28]. Thus, decreases in body weight and body fat are important and are the primary targets in obesity therapy [29,30]. The BTD-1E group showed significantly lower body weight compared with the db control group. This anti-hyperlipedemic effect was not associated with the inhibition of diet consumption because the BTD-1E group showed a slightly higher level of diet consumption compared with the db control group.
Body weight is usually related with lipid metabolism. Thus we analyzed the contents of serum lipid after BTD-1E treatment. In the present study, BTD-1E resulted in remarkably lower serum T-CHOL and LDL-C levels compared with the db control group. These results show that BTD-1E can regulate lipogenesis and lipolysis to maintain serum T-CHOL and LDL-C levels in the blood of the mice. It was reported that Monascus-fermented soybean extract reduced T-CHOL and LDL-C levels in rats fed a high cholesterol diet [31]. In several studies, a small increase of HDL-C is observed after feeding soybean or isoflavones [32], whereas in others no change or a small decrease in HDL-C is observed [33]. Yousef et al. reported HDL-C increased in a dose dependent manner [33]. This study was performed only for four weeks. A long term study is required to elucidate the mechanisms underlying the improvement effect on HDL-C levels by BTD-1E. TG levels usually change according to diet consumption. Since BTD-1E group showed slightly higher diet consumption compared with the db control group, thus TG levels of BTD-1E group slightly higher than db control group.
Lipid accumulation in adipocytes is determined by a balance of lipogenesis and lipolysis [34]. In this study, the liver weight of the BTD-1E group in relation to final body weight was significantly lower when compared with that of the db control group. In the liver, lipid droplets were obviously less common in the BTD-1E administered mice compared with the db control mice. Hepatomegaly and increases in the hepatic lipid accumulation are commonly encountered in obese and diabetic animals with hyperinsulinemia [34]. A long term study is required to explain clearly the mechanisms underlying the effect on organ weights, specially EPDF, by BTD-1E.
In conclusion, our results suggest that BTD-1E has the beneficial effect of preventing obesity in db/db mice. BTD-1E administration resulted in lower body weight, serum lipid levels, liver weight, and the deposition of neutral lipid in the liver without appetite modulation in db/db mice. BTD-1E thus might be used for prevention and/or therapy for overweight, obesity, and further cardiovascular diseases related with hyperlipidemia.
Figures and Tables
Figure 1
Effects of BTD-1E on body weight and diet intake. (A) body weight, and (B) dietary consumption after administration of BTD-1E in db/db mice. Data represent the means±SD (n=6). *P<0.05 as compared to the db control group.

Figure 2
Effects of BTD-1E on serum lipid profiles. (A) total cholesterol, (B) LDL cholesterol, (C) HDL-cholesterol, and (D) triglyceride levels. Data represent the means±SD (n=6). *P<0.05 and *P<0.01 as compared to the db control group.

Figure 3
Effects of BTD-1E on relative organ weight. (A) final body weight, (B) relative liver weight: (liver weight/final body weight) × 100%, (C) relative spleen weight, (D) relative testis weight, (E) relative kidney weight, and (F) relative epidermal fat weight. Data represent the means±SD (n=6). *P<0.05 and *P<0.01 as compared to the db control group.

Figure 4
Effect of BTD-1E on fat accumulation in the liver. (A) C57BL/6J, (B) db control, and (C) BTD-1E treated groups. Many fat droplets were diffusely present in the liver of the db control mice. In contrast, the fat droplets were remarkably decreased in the BTD-1E administered mice. Oil red O (red) and hematoxylin-eosin stain (blue).
Acknowledgments
This research was financially supported by the Ministry of Knowledge Economy (MKE) and Korea Institute for Advancement of Technology (KIAT) through the Supporting and servicing enterprises for regional industry, and by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0030750).
References
1. Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004. 291(23):2847–2850.
2. Strader CD, MacIntyre DE, Candelore MR, Parker EM. Molecular approaches to the discovery of new treatments for obesity. Curr Opin Chem Biol. 1997. 1(2):204–209.
3. Kim Y, Park YJ, Yang SO, Kim SH, Hyun SH, Cho S, Kim YS, Kwon DY, Cha YS, Chae S, Choi HK. Hypoxanthine levels in human urine serve as a screening indicator for the plasma total cholesterol and low-density lipoprotein modulation activities of fermented red pepper paste. Nutr Res. 2010. 30(7):455–461.
4. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999. 282(16):1523–1529.
5. Kopelman PG. Obesity as a medical problem. Nature. 2000. 404(6778):635–643.
6. Lew EA. Mortality and weight: insured lives and the American Cancer Society studies. Ann Intern Med. 1985. 103:1024–1029.
7. Kang NH, Lee WK, Yi BR, Park MA, Lee HR, Park SK, Hwang KA, Park HK, Choi KC. Modulation of lipid metabolism by mixtures of protamine and chitooligosaccharide through pancreatic lipase inhibitory activity in a rat model. Lab Anim Res. 2012. 28(1):31–38.
8. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007. 369(9555):71–77.
9. Bays HE. Current and investigational antiobesity agents and obesity therapeutic treatment targets. Obes Res. 2004. 12(8):1197–1211.
10. Hauptman JB, Jeunet FS, Hartmann D. Initial studies in humans with the novel gastrointestinal lipase inhibitor Ro 18-0647 (tetrahydrolipstatin). Am J Clin Nutr. 1992. 55:1 Suppl. 309S–313S.
11. McNeely W, Goa KL. Sibutramine. A review of its contribution to the management of obesity. Drugs. 1998. 56(6):1093–1124.
12. Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994. 350(2-3):240–244.
13. Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2003. 4:CD004094.
14. Wooltorton E. Obesity drug sibutramine (Meridia): hypertension and cardiac arrhythmias. CMAJ. 2002. 166(10):1307–1308.
15. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007. 369(9555):71–77.
16. Nakanishi H, Onose S, Kitahara E, Chumchuen S, Takasaki M, Konishi H, Kanekatsu R. Effect of environmental conditions on the α-glucosidase inhibitory activity of mulberry leaves. Biosci Biotechnol Biochem. 2011. 75(12):2293–2296.
17. Tsuduki T, Nakamura Y, Honma T, Nakagawa K, Kimura T, Ikeda I, Miyazawa T. Intake of 1-deoxynojirimycin suppresses lipid accumulation through activation of the beta-oxidation system in rat liver. J Agric Food Chem. 2009. 57(22):11024–11029.
18. Ezure Y, maruo S, Miyazaki K, kawamata M. Moranoline (1-Deoxynojirimycin) fermentation and its improvement. Agric Biol Chem. 1985. 49(4):1119–1125.
19. Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med. 1995. 18(4):303–311.
20. Inoue I, Shinoda Y, Nakano T, Sassa M, Goto S, Awata T, Komoda T, Katayama S. Acarbose ameliorates atherogenecity of low-density lipoprotein in patients with impaired glucose tolerance. Metabolism. 2006. 55(7):946–952.
21. Cheik NC, Rossi EA, Guerra RL, Tenorio NM, Oller do Nascimento CM, Viana FP, Manzoni MS, Carlos IZ, Leao da Silva P, Vendramini RC, Damaso AR. Effects of a ferment soy product on the adipocyte area reduction and dyslipidemia control in hypercholesterolemic adult male rats. Lipids Health Dis. 2008. 7:50.
22. Nojima H, Kimura I, Chen FJ, Sugihara Y, Haruno M, Kato A, Asano N. Antihyperglycemic effects of N-containing sugars from Xanthocercis zambesiaca, Morus bombycis, Aglaonema treubii, and Castanospermum australe in streptozotocin-diabetic mice. J Nat Prod. 1998. 61(3):397–400.
23. Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, Oita S, Oikawa S, Miyazawa T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. 2007. 55(14):5869–5874.
24. Kim HS, Lee JY, Hwang KY, Cho YS, Park YS, Kang KD, Seong SI. Isolation and identification of a Bacillus sp. producing alpha-glucosidase inhibitor 1-deoxynojirimycin. Korean J Microbiol Biotechnol. 2011. 39(1):49–55.
25. Chae JY, Lee JY, Hoang IS, Whangbo D, Choi PW, Lee WC, Kim JW, Kim SY, Choi SW, Rhee SJ. Analysis of functional components of leaves of different mulberry cultivars. J Korean Soc Food Sci Nutr. 2003. 32(1):15–21.
26. Hwang KY, Kim YH, Cho YS, Park YS, Lee JY, Kang KD, Kim K, Joo DK, Ahn DK, Seong SI. Hypoglycemic effect of fermented soybean culture mixed with mulberry leaves on neonatal streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2008. 37(4):452–458.
27. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995. 854:1–452.
28. DeLany JP, Blohm F, Truett AA, Scimeca JA, West DB. Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. Am J Physiol. 1999. 276(4):R1172–R1179.
29. Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005. 86(5):599–602.
30. Pyo YH, Seong KS. Hypolipidemic effects of Monascus-fermented soybean extracts in rats fed a high-fat and -cholesterol diet. J Agric Food Chem. 2009. 57(18):8617–8622.
31. Song T, Lee SO, Murphy PA, Hendrich S. Soy protein with or without isoflavones, soy germ and soy germ extract, and daidzein lessen plasma cholesterol levels in golden Syrian hamsters. Exp Biol Med (Maywood). 2003. 228(9):1063–1068.
32. Yousef MI, Kamel KI, Esmail AM, Baghdadi HH. Antioxidant activities and lipid lowering effects of isoflavone in male rabbits. Food Chem Toxicol. 2004. 42(9):1497–1503.
33. Azain MJ, Hausman DB, Kasser TR, Martin RJ. Effect of somatotropin and feed restriction on body composition and adipose metabolism in obese Zucker rats. Am J Physiol. 1995. 269(1):E137–E144.
34. Ikeda H, Shino A, Matsuo T, Iwatsuka H, Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes. 1981. 30(12):1045–1050.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download