Abstract
Altered expression of neurotrophic factors as well as neuroinflammation is commonly associated with Major depressive disorder (MDD) and Alzheimer's disease (AD). To investigate whether or not reserpine-induced MDD affects the expression of AD-related proteins, the expression of γ-secretase components and substrate were measured in brains of ICR mice following reserpine treatment for 15 days. In active avoidance test, total response time and peak slightly increased in the 2 mg/kg reserpine (RSP2)-treated group compared to vehicle-treated group (P<0.05). Expression and phosphorylation of MKP-1, which is a key factor in MDD pathology, were both higher in the RSP2-treated group than the vehicle- and 1 mg/kg reserpine (RSP1)-treated groups (P<0.02). Furthermore, full-length expression of amyloid precursor protein (APP) was enhanced in the RSP1 and RSP2-treated groups compared to the vehicle-treated group, whereas expression of γ-secretase components decreased (P<0.03). Among the three components of the γ-secretase complex, nicastrin protein underwent the largest decrease in expression, as detected by Western blotting (P<0.03). Therefore, the data presented here provide additional evidence about the pathological correlation between MDD and AD.
Major depressive disorder (MDD) is a severe psychiatric disorder that exhibits a large range of serious deficits on recollection memory, list learning, recall, verbal and visual memory, executive function, attention, and verbal fluency [1-3]. The etiology of MDD is related to altered regulation of monoamines, corticotropin-releasing hormone (CRH), and brain-derived neurotrophic factor (BDNF) expression [4-6].
Interestingly, several of the proposed mechanisms for MDD are similar to those for neurodegenerative disorders such as Alzheimer's disease (AD). Overdrive within the hypothalamic-pituitary-adrenal (HPA) axis as well as reduction of serotonin levels may contribute to the development of cognitive impairment in both MDD and AD [1]. Further, altered levels of neurotrophic factors play major roles in the development of MDD, i.e. impairment of BDNF signaling stimulates the amyloidogenic pathway, leading to activation of apoptosis in hippocampal neurons [7]. Furthermore, MDD and AD appear to share a common neuroinflammatory response. Increased levels of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12, and TNF-α have been observed in patients suffering from MDD and AD [8,9]. Regarding anti-inflammatory cytokines, reduced levels of IL-4 and IL-10 have been found in MDD patients and an animal model of AD, although results have shown an elevated anti-inflammatory response [10-12]. However, there has been no conclusive evidence as to whether or not the pathological and biochemical changes in MDD alter the expression of γ-secretase components, causing AD.
Therefore, the aim of this study was to investigate the effect of MDD induced by reserpine on amyloid precursor protein (APP) and the γ-secretase components expression. To achieve this, the expression of these proteins were detected in the brain of ICR mice treated reserpine for 15 days. The present study using MDD animal model demonstrates that the pathological and biochemical alterations in reserpine-induced MDD stimulate inhibition of APP cleavage by downregulating the expression of γ-secretase components.
The animal protocol used in this study was reviewed and approved based on the ethical procedures of the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2011-000220). Adult ICR mice were purchased from SamTacho (Osan, Korea) and handled at the Pusan National University Laboratory Animal Resources Center according to National Institutes of Health guidelines. All mice were given a standard irradiated chow diet (Purina Mills, Seoungnam, Korea) ad libitum and were maintained in a specific pathogen-free state under a strict light cycle (light on at 06:00 h and off at 18:00 h) at a temperature of 22±2℃ and at 40-60% relative humidity.
Ten-week-old ICR mice (n=21) were assigned to one of three groups (n=7 per group): a vehicle-treated group, reserpine (1 mg/kg)(RSP1)-treated group, and reserpine (2 mg/kg) (RSP2)-treated group. The vehicle-treated group received a consistent volume of distilled water daily via intraperitoneal injection, whereas the treatment groups received 1 or 2 mg/kg of reserpine for 15 days via intraperitoneal injection. At 15 days after commencement of reserpine and vehicle treatment, all animals were immediately sacrificed using CO2 gas to acquire brain tissues, which were stored in Eppendorf tubes at -70℃ until assayed.
Proteins from brain tissues of vehicle- and reserpine-treated mice were separated by 4-20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 2 h, after which the resolved proteins were transferred to nitrocellulose membranes for 2 h at 40 V. Each membrane was incubated separately with primary antibody: anti-MKP-1 (SC-1199, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p-MKP-1 antibody (ab78898, Abcam, Cambridge, MA, UK), anti-human Pen-2 antibody (SC-32946, Santa Cruz Biotechnology, CA, USA), anti-PS-2 antibody (#21925, Cell Signaling Technology, Boston, MA, USA), anti-APP antibody (A8717, Sigma-Aldrich, St. Louis, MO, USA), anti-APH-1 antibody (A9603, Sigma-Aldrich, St. Louis, MO, USA), or anti-actin antibody (A5316, Sigma-Aldrich, St. Louis, MO, USA) overnight at 4℃. The membranes were then washed with washing buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and 0.05% Tween 20) and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA, USA) diluted 1:1,000 at room temperature for 2 h. The membrane blots were developed using Chemiluminescence Reagent Plus kits (Pfizer, New York, NY, USA).
Active avoidance test was performed according to the method described by Banasr et al. [13] in a learned helplessness chamber composed of Active Avoidance Shuttle Box (UGO BASILE, Collegeville, USA). After habituation for 5 min, mice received 30 randomized escapable footshocks at an intensity of 0.25 mA. In each shock trial, the gate separating the two halves of the shuttle box was opened 5 s before shock onset and remained open for the duration of the shock. The average intertrial interval was 30 s (range 20-100 s). The first five trials required one crossing to terminate footshock (FR1), whereas the remaining 15 trials required two crossings (FR2). Experimental results are indicated as total time and each peak for response.
Before investigating the effect of MDD on the expression of γ-secretase components, MDD in ICR mice was induced by reserpine administration for 15 days. To confirm reserpine-induced MDD in ICR mice, alteration of mice behavior was detected using an active avoidance test. Total response time was significantly longer in the RSP2-treated group compared to the vehicle-treated group, whereas the response of the RSP1-treated group was not affected (Figure 1A). Further, long response peaks were often observed in the RSP2-treated group compared to the vehicle- or RSP1-treated group (Figure 1B). Therefore, reserpine administration for 15 days induced MDD-like behavior at a concentration of 2 mg/kg, as reported in a previous study.
MKP-1 has been identified as a key factor in the pathophysiology of MDD and has become a new therapeutic target [14]. To investigate whether or not reserpine treatment induces MDD in brains of ICR mice, the expression and phosphorylation levels of MKP-1 were measured in the brain cortex of ICR mice following reserpine treatment using an MKP-1-specific antibody. The highest level of MKP-1 expression was observed in the RSP2-treated group, whereas the RSP1-and vehicle-treated groups maintained their levels of MKP-1 expression (Figure 2). In addition, a similar pattern of p-MKP-1 was observed. Significant enhancement of MKP-1 phosphorylation was detected in the RSP2-treated group, whereas there was no change in the RSP1-treated group (Figure 2). These results indicate that RSP2 treatment successfully induced the biochemical phenotype of MDD in the brain cortex of ICR mice.
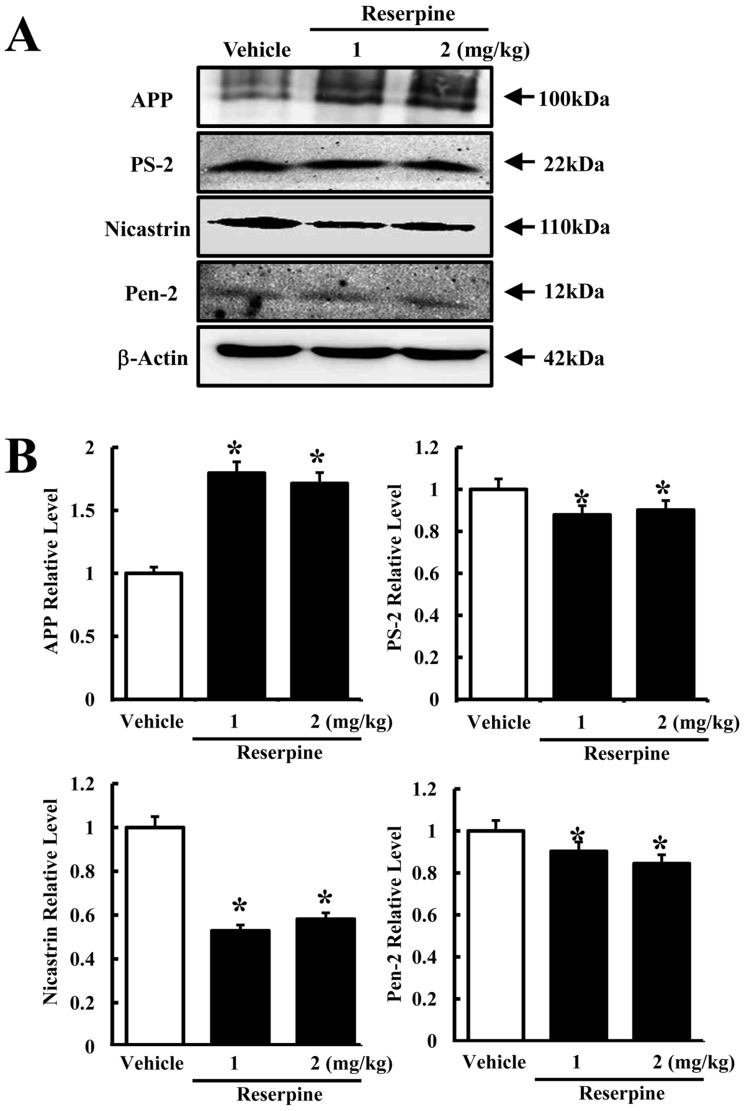
To determine whether or not reserpine-induced MDD alters the expression of γ-secretase components, the expression levels of γ-secretase-related factors were measured in the brain cortex of reserpine-induced MDD mice. In the γ-secretase substrate analysis, the expression of full-length APP increased in both reserpine-treated groups compared to control (Figure 3B). However, the expression of all three γ-secretase components decreased in the RSP1- and RSP2-treated groups, although the decrease ratio varied. Especially, a dramatic decrease in nicastrin expression was detected regardless of the reserpine dose. The level of PS-2 expression slightly decreased in the RSP1- and RSP2-treated groups as Pen-2 expression (Figure 3B). These results suggest that reserpine-induced MDD may enhance the suppression of APP cleavage by inhibiting expression of γ-secretase components.
AD is the most common neurodegenerative disorder associated with dementia and affects approximately 7-10% of humans over the age of 65 [4]. Mutations in the genes expressing APP, presenilin-1 (Ps-1), and presenilin-2 (Ps-2) have been identified as genetic factors of AD [15,16]. Such genetic factors induce AD by increasing the accumulation and aggregation of Aβ peptides and neurofibrilary tangles (NFTs) [17,18]. Meanwhile, AD and MDD have several symptoms in common based on comparable pathophysiological features and genetic predisposition. In this study, we provide novel evidence supporting a connection between AD and MDD.
Reserpine is an FDA-approved indole alkaloid antipsychotic and antihypertensive drug that is most often applied to the regulation of high blood pressure and the relief of psychotic symptoms [19]. Recently, the pharmacological efficacy of this drug was investigated in a transgenic C. elegans model of AD [20,21]. Reserpine treatment was found to extend the lifespan of the human Aβ-expressing transgenic C. elegans model. Further, the Aβ-expressing transgenic model treated with resperine survived for a long time under thermal stress conditions with no alteration in total Aβ concentration [19]. However, in this study, reserpine was used to induce MDD in ICR mice. As shown in Figure 3, reserpine-induced MDD induced a decrease in APP cleavage by inhibiting the expression of γ-secretase components.
Until now, there is no hard conclusive evidence as to if a γ-secretase is directly related to MDD. But, some reports suggested the indirect proof for this correlation. A broad spectrum of antidepressants has been used for AD treatment. Especially, imipramine having ability of the tricyclic antidepressant or the selective serotonin reuptake inhibitor citalopram stimulated the enhanced APP production by 3.2 or 3.4-fold, while only imipramine increased the protein kinaes C (PKC) level [22]. However, our study has been used MDD inducer, reserpine, to investigate the correlation between MDD and γ-secretase function. Full length APP expression was significantly increased by reserpine treatment.
Furthermore, two PS-1 varients (A431E and L235V) increased the incidence of clinical depression by the induction of monoamine oxidase-A (MAO-A) activity [23]. But, our study was focused on the level of PS-2 expression in the brain of MDD animal model. Further study will be required to identify the effect of reserpine-induced MDD on MAO-A activity
Taken together, our results provide novel evidence of a correlation between MDD and AD. Moreover, these findings greatly contribute to the development of a therapeutic drug through the inhibition of γ-secretase activity and identify the common factors regulating MDD and AD.
References
1. Sierksma AS, van den Hove DL, Steinbusch HW, Prickaerts J. Major depression, cognitive dysfunction and Alzheimer's disease: is there a link? Eur J Pharmacol. 2010; 626(1):72–82. PMID: 19837053.

2. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001; 178:200–206. PMID: 11230029.

3. Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008; 106(1-2):1–27. PMID: 17707915.

4. Wuwongse S, Chang RC, Law AC. The putative neurodegenerative links between depression and Alzheimer's disease. Prog Neurobiol. 2010; 91(4):362–375. PMID: 20441786.

5. Haavik J, Blau N, Thony B. Mutations in human monoamine-related neurotransmitter pathway genes. Hum Mutat. 2008; 29(7):891–902. PMID: 18444257.

6. Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009; 164(1):300–330. PMID: 19358877.

7. Matrone C, Ciotti MT, Mercanti D, Marolda R, Calissano P. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proc Natl Acad Sci USA. 2008; 105(35):13139–13144. PMID: 18728191.
8. Leonard BE, Myint A. Inflammation and depression: is there a causal connection with dementia? Neurotox Res. 2006; 10(2):149–160. PMID: 17062376.

9. Guerreiro RJ, Santana I, Brás JM, Santiago B, Paiva A, Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimer's disease and mild cognitive impairment. Neurodegener Dis. 2007; 4(6):406–412. PMID: 17934323.

10. Richartz-Salzburger E, Batra A, Stransky E, Laske C, Kohler N, Bartels M, Buchkremer G, Schott K. Altered lymphocyte distribution in Alzheimer's disease. J Psychiatr Res. 2007; 41(1-2):174–178. PMID: 16516234.
11. Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009; 210(1-2):3–12. PMID: 19269040.
12. Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009; 42(5):182–188. PMID: 19724980.

13. Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010; 15(5):501–511. PMID: 18825147.

14. Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010; 16(11):1328–1332. PMID: 20953200.

15. Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995; 269(5226):973–977. PMID: 7638622.

16. Van Broeckhoven C, Backhovens H, Cruts M, De Winter G, Bruyland M, Cras P, Martin JJ. Mapping of a gene predisposing to early-onset Alzheimer's disease to chromosome 14q24.3. Nat Genet. 1992; 2(4):335–339. PMID: 1303290.

17. Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001; 81(2):741–766. PMID: 11274343.

18. Williamson J, Goldman J, Marder KS. Genetic aspects of Alzheimer disease. Neurologist. 2009; 15(2):80–86. PMID: 19276785.

19. Arya U, Dwivedi H, Subramaniam JR. Reserpine ameliorates Abeta toxicity in the Alzheimer's disease model in Caenorhabditis elegans. Exp Gerontol. 2009; 44(6-7):462–466. PMID: 19264117.
20. Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006; 313(5793):1604–1610. PMID: 16902091.

21. Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008; 7(3):394–404. PMID: 18331616.

22. Pakaski M, Bjelik A, Hugyecz M, Kasa P, Janka Z, Kalman J. ipramine and citalopram facilitate amyloid precursor protein secretion in vitro. Neurochem Int. 2005; 47(3):190–195. PMID: 15955598.
23. Pennington PR, Wei Z, Rui L, Doig JA, Graham B, Kuski K, Gabriel GG, Mousseau DD. Alzheimer disease-related presenilin-1 variants exert distinct effects on monoamine oxidase-A activity in vitro. J Neural Transm. 2011; 118(7):987–995. PMID: 21373759.
Figure 1
Behavioral changes in reserpine-induced MDD mice. ICR mice were administrated saline or reserpine (1 or 2 mg/kg) for 15 days. Altered behavioral performance in ICR mice was measured using active avoidance test as described in Materials and Methods. (A) Total response time was calculated from 30 randomized escapable footshocks, each at an intensity of 0.25 mA. (B) Response peak indicates the time spent in one half of the shuttle boxes. Data represent the mean±SD from three replicates. *P<0.05 is the significance level compared to the vehicle-treated group.
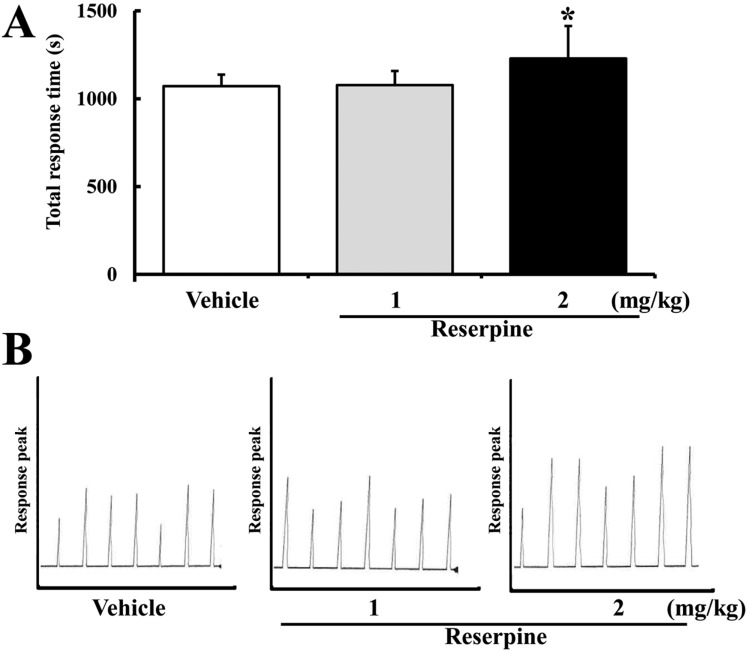
Figure 2
Expression of total and phosphorylated MKP-1 in brain cortex of reserpine-induced MDD mice. The cortex region was prepared from brain tissues of vehicle- and reserpine-treated mice. Fifty micrograms of protein per sample was immunoblotted with antibody for each protein. Expression levels of total and phosphorylated MKP-1 were detected using anti-MKP-1 and p-MKP-1 primary antibodies and horseradise peroxidase-conjugated goat anti-rabbit IgG (A). The intensity of each protein was calculated using an imaging densitometer (B). Data represent the mean¡¾SD from three replicates. *P<0.05 is the significance level compared to the vehicle-treated group.
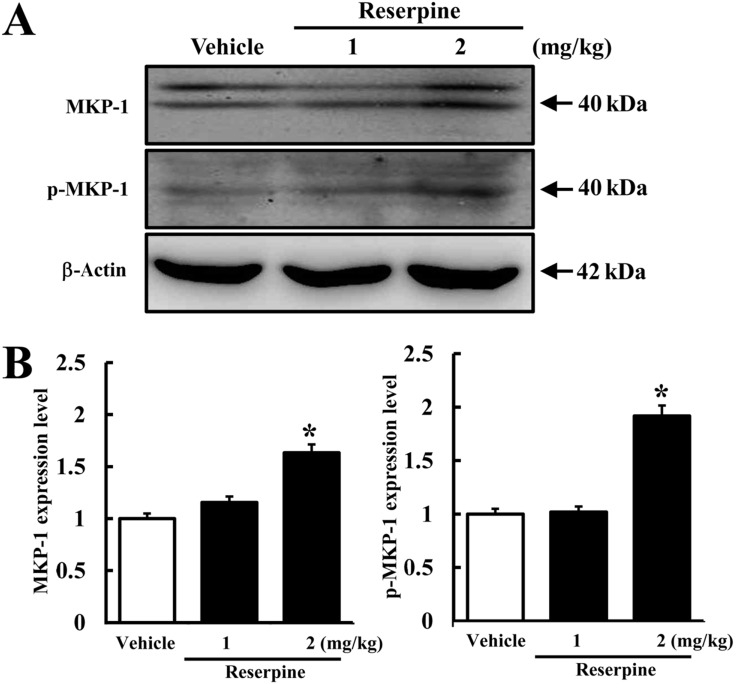
Figure 3
Expression of γ-secretase components in brain tissues of reserpine-induced MDD mice. The cortex region was prepared from brain tissues of vehicle- and reserpine-treated mice. Fifty micrograms of protein per sample was immunoblotted with antibody for each protein. Expression levels of full-length APP and γ-secretase components were detected using specific antibody and horseradish peroxidase-conjugated goat anti-rabbit IgG (A). The intensity of each protein was calculated using an imaging densitometer (B). Data represent the mean¡¾SD from three replicates. *P<0.05 is the significance level compared to the vehicle-treated group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download