Abstract
Exercise training can improve strength and lead to adaptations in the skeletal muscle and nervous systems. Skeletal muscles can develop into two types: fast and slow, depending on the expression pattern of myosin heavy chain (MHC) isoforms. Previous studies reported that exercise altered the distribution of muscle fiber types. It is not currently known what changes in the expression of caveolins and types of muscle fiber occur in response to the intensity of exercise. This study determined the changes in expression of caveolins and MHC type after forced exercise in muscular and non-muscular tissues in rats. A control (Con) group to which forced exercise was not applied and an exercise (Ex) group to which forced exercise was applied. Forced exercise, using a treadmill, was introduced at a speed of 25 m/min for 30 min, 3 times/day (07:00, 15:00, 23:00). Homogenized tissues were applied to extract of total RNA for further gene analysis. The expression of caveolin-3 and MHC2a in the gastrocnemius muscle of female rats significantly increased in the Ex group compared with the Con group (P<0.05). Furthermore, in the gastrocnemius muscle of male rats, the expression of MHC2x was significantly different between the two groups (P<0.05). There was an increased expression in caveolin-3 and a slightly decreased expression in TGFβ-1 in muscular tissues implicating caveolin-3 influences the expression of MHC isoforms and TGFβ-1 expression. Eventually, it implicates that caveolin-3 has positive regulatory function in muscle atrophy induced by neural dysfunction with spinal cord injury or stroke.
Endurance exercise has many health benefits, including improvements in muscle metabolism, cardiovascular function, and increased exercise tolerance, and it also induces mitochondrial biogenesis in skeletal muscle [1]. It is well documented that exercise induces several physiological and biochemical changes in the brain [2], and it improves exercise tolerance, endothelial function, and the biochemical and structural parameters of skeletal muscles [3]. Regarding neuromuscular junction (NMJ) changes induced by exercise, it has been reported that exercise affects neither the number and distribution of acetylcholine receptors (AChR) nor the specific activities of choline acetyltransferase (CAT) and acetylcholinesterase (AChE) [4].
Exercise training induces alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent animals [5], including alterations in antioxidant mechanisms [6] and the induction of hypertrophy [7]. Depending on the muscle type and the nature of the exercise, different studies have reported increases in oxidative capacity [8], the number and size of mitochondria [9], muscle weight [10], the appearance of split muscle fibers [11], and an altered distribution of muscle fiber types [4].
Skeletal muscles are one of the most important components responsible for physical performance and adaptation to exercise [12]. They can be characterized into functionally distinct slow (type I) and fast (type II) muscles, according to the expression pattern of myosin heavy chain (MHC) isoforms in the fibers. The MHC gene family consists of eight isoforms and four types are expressed in adult skeletal muscle: slow MHC and fast MHC, of which there are three isoforms, 2a, 2x, and 2b (or MHC2A, MHC2X, and MHC2B) [13]. The three adult fast MHC isoforms are expressed in different types of skeletal muscle fibers and have different physiological characteristics, with 2A fibers being smaller, slower, and more oxidative, 2B fibers typically being the largest, fastest, and most glycolytic, and 2X or 2D/X fibers falling between these extremes [14]. Physical activity, including exercise, can alter the properties of MHC isoforms and change the isoform content [15].
It is well known that exercise can have beneficial effects on insulin resistance, through the activation of glucose transporters, especially caveolin-1, which plays an important role in glucose uptake in L6 skeletal muscle cells [16]. Caveolins are integral membrane proteins that play a role in essential cellular functions. They normally have two main functions: intracellular transport of signaling molecules and transmitters [17]. The caveolin gene family consists of caveolin-1, -2, and -3. Caveolin-1 has been reported to interact with various intracellular signaling molecules, including growth factors, such as the epidermal growth factor receptor (EGFR) [18] and estrogen receptor (ER) [19]. Caveolin-3 is expressed in a muscle-specific manner and mutations in caveolin-3 cause limb girdle muscular dystrophy, leading to apoptosis of skeletal muscle [20]. Loss of caveolin-3 resulting from dominant-negative mutations in the caveolin-3 gene causes autosomal dominant limb-girdle muscular dystrophy 1C (LGMD1C).
Myostatin is a member of the transforming growth factor beta (TGFβ) superfamily and plays an important role in the negative regulation of skeletal muscle volume [21]. Over-expression of myostatin causes severe muscular atrophy [22,23], whereas targeted disruption of myostatin markedly increases muscle mass in mice [21,24]. Recently, caveolin-1 was reported to inhibit the activation of the type I receptor for TGFβ-1, which induces growth arrest in non-muscle cells [25]. Accordingly, an increase in myostatin activity, resulting from loss of caveolin-3 in muscle, might participate in the pathogenesis of skeletal muscle atrophy in LGMD1C patients [26]. However, it remains unclear how caveolin gene expression changes after forced exercise and the relevance of such on shifts on the type of MHC isoforms. Thus, the purpose of this study was to investigate the changes of expression of caveolins, MHC type, and TGFβ-1 after forced exercise, using treadmills, in muscular and non-muscular tissues.
The materials and chemicals used in this study, including Tri-reagent, were obtained from company (Invitrogen, Carlsbad, CA, USA).
Sprague Dawley (SD) rat males (n=16) and females (n=16), 10-12 weeks of age, and between 290-300 g were used. The animals were housed in an air-conditioned room with a constant temperature of 22±2℃ with free access to food and water, and subject to a photoperiod of 12 h of light and 12 h of darkness. All animal experiments were performed according to a protocol set out in the guidelines of the Animal Experiments Ethics Committee at Inje University (Approval No. 2009-083).
All experimental rats performed the forced exercise over a period of 4 weeks using treadmills. The protocol for treadmill exercise is as follows: the speed of the treadmill was 25 m/min, with a frequency of 6 days per week, for 30 min. The rat was exercised three times a day, 07:00, 15:00, 23:00 (8 h intervals).
Brain (fore, mid, and hind) tissue, heart, kidneys (from females) and skeletal muscles (gastrocnemius and soleus, from males and females) were homogenized in 1 mL of Tri-Reagent, and total RNA was extracted according to the manufacturer's instructions. Total RNA was treated with RNase-free distilled water, and then reverse transcribed by reverse transcriptase and oligo-dT primers in a Px2 Thermal cycler (Thermo Electron Co., Waltham, MA, USA), using the RT-PCR method. Primers were used as following : caveolin isoforms (cav-1, -2, and -3), MHC isoforms (MHC-1β, MHC-2a, -2x, and -2b), and TGFβ-1. The primers were sequenced to confirm the specificity of amplification and the sequences are shown in Table 1.
Data were collected from repeated experiments and are presented as mean±SEM. Independent T-test was used to compare the significant difference of gene expression in each group. A P value less than 0.05 denoted significant differences. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) ver.16 software (IBM, New York, NY, USA).
To determine how the expression of caveolin isoform genes changed in non-muscular and skeletal muscular tissue, which type(s) of MHC genes were expressed in the muscles, and the changes in MHC gene expression and TGFβ-1 after forced exercise, we analyzed the expression of caveolin isoforms, MHC-1β, MHC-2a, -2x, -2b, and TGFβ-1 by RT-PCR. The RT-PCR results were semi-quantitatively analyzed using a densitometric method.
Cav-1 and -2 expression was slightly increased in the forebrain in the Ex group, compared with the Con group; however, cav-3 expression decreased in the Ex group (Figure 1-A). In the mid-brain, cav-1, -2, and -3 expression was slightly decreased in the Ex group, compared with the Con group (Figure 1-B). In the hind-brain, expression of cav-1 was increased in the Ex group, compared with the Con group, whereas expression of cav-2 in both groups was similar. Expression of cav-3 was slightly decreased in the Ex group, compared with the Con group. Stable expression of cav-3 was detected in all brain tissues (Figure 1-C).
Expression of caveolin genes in the heart and kidneys was analyzed after forced exercise. Cav-1 expression in the heart was slightly decreased in the Ex group, compared with the Con group; however cav-2 and -3 expression was slightly increased in the Ex group (Figure 2-A). In the kidneys, cav-1 and -2 expression was slightly decreased in the Ex group, compared with the Con group, while cav-3 expression was increased in the Ex group, compared with the Con group (Figure 2-B). Stable expression of cav-3 was detected in both the heart and kidneys.
Expression of caveolin genes was analyzed in the gastrocnemius tissue of male and female rats after forced exercise. In the gastrocnemius of male rats, expression of cav-1, -2, and -3 increased in the Ex group, compared with the Con group, but it was not significantly different (Figure 3-A). In female rats, cav-1 expression increased in the Ex group, compared with the Con group; however, there was no difference in cav-2 expression between the groups. Cav-3 expression was significantly increased in the Ex group, compared with the Con group (P<0.05; Figure 3-B).
In the soleus of male rats, cav-1 and -2 expression was decreased in the Ex group, compared with the Con group; however, there was no difference in cav-3 expression between the groups (Figure 4-A). In female rats, cav-1 and -2 expression was increased in the Ex group, compared with the Con group; however, there was no difference in cav-3 expression between the groups (Figure 4-B).
In male rats, MHC-2x expression was significantly increased in the Ex group compared to the Con group (P<0.05), whereas, there was no significant difference in MHC-1β, MHC-2a, and -2b expression between both groups (Figure 5-A). In female rats, MHC-2a expression was significantly increased in the Ex group, compared with the Con group (P<0.05); however, there were no significant differences in MHC-1β, MHC-2x, and -2b expression between the two groups (Figure 5-B). In both male and female rats, TGFβ-1 expression was slightly decreased, but not significantly so.
To determine which types of MHC genes were expressed in the muscles, and the change in expression of MHC genes and TGFβ-1, we analyzed the expression patterns of MHC-1β, MHC-2a, -2x, -2b, and TGFβ-1 in gastrocnemius muscle by RT-PCR. There was no significant difference in MHC-1β, MHC-2a, -2x, or -2b expression between the groups in male rats (Figure 6-A). Additionally, there was no significant difference in MHC-1β, MHC-2a, -2x, or -2b expression between the groups in female rats (Figure 6-B). In male and female rats, TGFβ-1 expression was slightly decreased, but not significantly so.
The present study was designed to determine the expression of caveolins in non-muscular and muscular tissue and the distribution of MHC isoforms in muscular tissue after forced exercise, and to reveal the relationship between caveolins and MHC isoform expression. Therefore, forced exercise was applied to SD rats, and the results were analyzed.
First, cav-1 and -2 expression differed between non-muscular and muscular tissues after forced exercise, and caveolin isoforms were differentially expressed in different tissues. Previous studies reported that caveolin-1 and -2 are co-expressed in many cell types, including adipocytes, fibroblasts, and endothelial cells [27], while the expression of caveolin-3 is muscle-specific [28]. Stable expression of cav-3 was detected in all tissues, including the brain. More recently, caveolin-1, -2, and -3 expression was identified in the brain [29]. Caveolin-1 and -2 are widely expressed in brain microvessels, endothelial cells, astrocytes, oligodendrocytes, Schwann cells, dorsal root ganglia, and hippocampal neurons. Caveolin-3 is also prominently expressed in astroglial cells [30-32].
Interestingly, the expression of cav-3 increased significantly in the Ex group, compared with the Con group, in the gastrocnemius of female rats. In the gastrocnemius of male rats, the expression of MHC-2x increased significantly in the Ex group, compared with the Con group. Moreover, in the gastrocnemius of female rats, the expression of MHC-2a was significantly increased in the Ex group compared to the Con group. These results suggest that forced exercise may induce shifts between the MHC-2a and -2x isoforms in the gastrocnemius of male and female rats. In the gastrocnemius of female rats, it appears that increased cav-3 expression was related to increased MHC-2a expression, as seen in the Ex group compared with the Con group.
Increased neuromuscular activity results in MHC isoform shifts from fast to slow muscle fibers [33], and inactivity results in a general shift in MHC expression and metabolic properties along a progression, from 1→2A→2X→2B [34]. Reduced synthesis rates of MHC are also observed [35], and mitochondrial dysfunction, which may arise as a result of oxidative stress, together with a decline in glycolytic enzyme activity, may lead to decreased energy production in aging skeletal muscles [36]. Furthermore, the paralyzed muscles of individuals with chronic traumatic spinal cord injury (SCI) are characterized by a high distribution of "fast" muscle fibers (type II), and in some instances by the complete exclusion of any slow fibers in what would normally be mixed fiber type muscles. Thus, MHC isoform expression appears to be altered by an increase in MHC II [37,38]. Oxidative stress, such as by nitric oxide (NO), down regulates caveolin-3 levels, due to an alteration in the DNA-binding activity of the muscle transcription factor myogenin, resulting in cachexia [39], while endurance training promotes a shift in the opposite direction, 2B→2X→2A→1 [34]. Many previous studies have reported the effects of exercise on changes in muscle fiber type [1,40-42].
In our results, the expression patterns of cav-3 and TGFβ-1 were contrary to each other in the gastrocnemius, and the gastrocnemius of male rats showed an increased expression of MHC2x in the Ex group, compared with the Con group. The gastrocnemius of female rats showed an increased expression of MHC2a in the Ex group, compared with the Con group. These results suggest that caveolin-3 may regulate shifts in the expression of MHC isoforms by inhibiting the expression of TGFβ-1. Further studies are needed to investigate how caveolins are related to the physiology of skeletal muscles and the mechanism of interaction between caveolins and TGFβ-1 in shifts in MHC type after physical exercise.
In summary, to investigate that pattern of caveolins and MHC isoforms expression, the relevance of changes of expression in caveolins and MHC isoforms, and influence of caveolin-3 to TGFβ-1 expression, male and female SD rat were applied forced exercise through treadmill running. We obtained the following results: The expression of MHC-2x was increased significantly in gastrocnemius of male-exercise group compared with the male-contol group. Also, the expression of caveolin-3 and MHC-2a were increased significantly in gastrocnemius of female-exercise group compared with the female-conrol group. Also there was a slightly decreased expression in TGFβ-1 in all muscular tissue. These results indicate that caveolin-3 may influence the expression of MHC isoforms and TGFβ-1 expression and prevent atrophy in skeletal muscle. Finally, it is necessary to understand physiology of muscular system in molecular level, in order to develop protocol of therapeutic exercise adjusted pathologic states.
Figures and Tables
Figure 1
Expression of caveolin-1, -2, and -3 in the brain of female rats. (A) fore-brain, (B) mid-brain, and (C) hind-brain. Con: non-treadmill exercised group, Ex: treadmill exercised group.
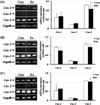
Figure 2
Expression of caveolin-1, -2, and -3 in the heart and kidney of female rats. (A) heart, (B) kidney. Con: non-treadmill exercised group, Ex: treadmill exercised group.
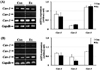
Figure 3
Expression of caveolin-1, -2, and -3 in the gastrocnemius muscle of male and female rats. (A) male, (B) female. Con: non-treadmill exercised group, Ex: treadmill exercised group.
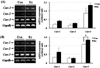
Figure 4
Expression of caveolin-1, -2, and -3 in the soleus muscle of male and female rats. (A) male, (B) female. Con: non-treadmill exercised group, Ex: treadmill exercised group.
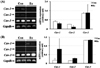
Figure 5
The expression of MHC-1β, MHC-2a, -2x, and -2b, and TGFβ-1 in the gastrocnemius muscle of male and female rats. (A) male, (B) female. Con: non-treadmill exercised group, Ex: treadmill exercised group.

Acknowledgments
The study was funded by the KRIBB Research Initiative Program (KGM0501113 to Y. Hong); BioGreen 21 Program (Code No. 20110301-061-542-03-00 to Y. Hong), Rural Development Administration, Republic of Korea.
References
1. Hood DA, Saleem A. Exercise-induced mitochondrial biogenesis in skeletal muscle. Nutr Metab Cardiovasc Dis. 2007. 17(5):332–337.
2. Sharma HS, Cervós-Navarro J, Dey PK. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neuroscience Research. 1991. 10(3):211–221.
3. Medeiros A, Vanzelli AS, Rosa KT, Irigoyen MC, Brum PC. Effect of exercise training and carvedilol treatment on cardiac function and structure in mice with sympathetic hyperactivity-induced heart failure. Braz J Med Biol Res. 2008. 41(9):812–817.
4. Kovanen V, Suominen H. Effects of age and life-time physical training on fiber composition of slow and fast skeletal muscle in rats. Pflugers Arch. 1987. 408(6):543–551.
5. Hammeren J, Powers S, Lawler J, Criswell D, Martin D, Lowenthal D, Pollock M. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int J Sports Med. 1992. 13(5):412–416.
6. Moran M, Delgado J, Gonzalez B, Manso R, Megías A. Responses of rat myocardial antioxidant defences and heat shock protein HSP72 induced by 12 and 24 week treadmill training. Acta Physiol Scand. 2004. 180(2):157–166.
7. Narici MV, Reeves ND, Morse CI, Maganaris CN. Muscular adaptations to resistance exercise in the elderly. J Musculoskelet Neuronal Interact. 2004. 4(2):161–164.
8. Silbermann M, Finkelbrand S, Weiss A, Gershon D, Reznick A. Morphometric analysis of aging skeletal muscle following endurance training. Muscle Nerve. 1983. 6(2):136–142.
9. Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. 1985. 403(4):369–376.
10. Wernig A, Irintchev A, Weisshaupt P. Muscle injury, cross-sectional area, and fiber type distribution in mouse soleus after intermittent wheel-running. J Physiol. 1990. 428:639–652.
11. Irintchev A, Wernig A. Muscle damage and repair in voluntary running mice: Strain and muscle differences. Cell Tissue Res. 1987. 249(3):509–521.
12. Pette D. Training effects on the contractile apparatus. Acta Physiol Scand. 1998. 162(3):367–376.
13. Allen DL, Sartorius CA, Sycuro LK, Leinwand LA. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem. 2001. 276(47):43524–43533.
14. Talmadge RJ, Roy RR, Edgerton VR. Muscle fiber types and function. Curr Opin Rheumatol. 1993. 5(6):695–705.
15. Braith RW, Magyari PM, Pierce GL, Edwards DG, Hill JA, White LJ, Aranda JM Jr. Effects of resistance exercise on skeletal muscle myopathy in heart transplant recipients. Am J Cardiol. 2005. 95(10):1192–1198.
16. Oh YS, Kim HJ, Ryu SJ, Cho KA, Park YS, Park H, Kim M, Kim CK, Park SC. Exercise type and muscle fiber specific induction of caveolin-1 expression for insulin sensitivity of skeletal muscle. Exp Mol Med. 2007. 39(3):395–401.
17. Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: A signalling hypothesis. Trends Cell Biol. 1994. 4(7):231–235.
18. Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997. 272(48):30429–30438.
19. Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol Endocrinol. 2002. 16(1):100–115.
20. Capanni C, Sabatelli P, Mattioli E, Ognibene A, Columbaro M, Lattanzi G, Merlini L, Minetti C, Maraldi NM, Squarzoni S. Dysferlin in a hyperCKaemic patient with caveolin 3 mutation and in C2C12 cells after p38 MAP kinase inhibition. Exp Mol Med. 2003. 35(6):538–544.
21. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997. 387(6628):83–90.
22. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002. 296(5572):1486–1488.
23. Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003. 285(4):E876–E888.
24. Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis. 2003. 35(4):227–238.
25. Razani B, Zhang XL, Bitzer M, von Gersdorff G, Böttinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001. 276(9):6727–6738.
26. Ohsawa Y, Hagiwara H, Nakatani M, Yasue A, Moriyama K, Murakami T, Tsuchida K, Noji S, Sunada Y. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest. 2006. 116(11):2924–2934.
27. Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000. 275(28):21605–21617.
28. Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996. 271(25):15160–15165.
29. Cameron PL, Ruffin JW, Bollag R, Rasmussen H, Cameron RS. Identification of caveolin and caveolin-related proteins in the brain. J Neurosci. 1997. 17(24):9520–9535.
30. Ikezu T, Ueda H, Trapp BD, Nishiyama K, Sha JF, Volonte D, Galbiati F, Byrd AL, Bassell G, Serizawa H, Lane WS, Lisanti MP, Okamoto T. Affinity-purification and characterization of caveolins from the brain: Differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998. 804(2):177–192.
31. Bu J, Bruckner SR, Sengoku T, Geddes JW, Estus S. Glutamate regulates caveolin expression in rat hippocampal neurons. J Neurosci Res. 2003. 72(2):185–190.
32. Arvanitis DN, Wang H, Bagshaw RD, Callahan JW, Boggs JM. Membrane-associated estrogen receptor and caveolin-1 are present in central nervous system myelin and oligodendrocyte plasma membranes. J Neurosci Res. 2004. 75(5):603–613.
33. Pette D, Staron RS. Mammalian skeletal muscle fiber type transition. Int Rev Cytol. 1997. 170:143–223.
34. Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve. 2000. 23(5):661–679.
35. D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003. 552(Pt 2):499–511.
36. Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med. 2003. 35(1):1–8.
37. Round JM, Barr FM, Moffat B, Jones DA. Fibre areas and histochemical fibre types in the quadriceps muscle of paraplegic subjects. J Neurol Sci. 1993. 116(2):207–211.
38. Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia. 1995. 33(9):514–522.
39. Martinez-Moreno M, Martinez-Ruiz A, Alvarez-Barrientos A, Gavilanes F, Lamas S, Rodriguez-Crespo I. Nitric oxide downregulates caveolin-3 levels through the interaction with myogenin, its transcription factor. J Biol Chem. 2007. 282(32):23044–23054.
40. Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol. 1997. 83(1):59–66.
41. Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001. 8(6):1046–1056.
42. Demetrovics Z, Kurimay T. Exercise addiction: a literature review. Psychiatr Hung. 2008. 23(2):129–141.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download