Materials and Methods
Materials
Animals
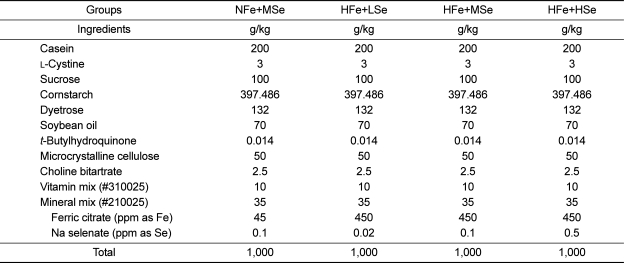
Experimental diets
Experimental designs
Sample collection
Blood analysis
Fe and Se analyses in the liver
Tumor incidence
Immunohistochemistry of proliferating cell nuclear antigen (PCNA)
TUNEL assay
Glutathione peroxidase (GPx) activity
Western blot analysis
Determination of hepatic malondialdehyde (MDA)
Statistical analysis
Results
Changes in body weights
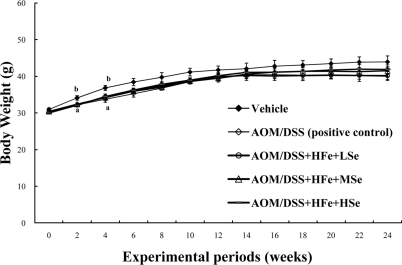 | Figure 1Change in the body weights of mice fed the high Fe diet. All AOM/DSS-treated groups showed a low body weight compared with the vehicle group during experimental periods. AOM: azoxymethane, DSS: dextran sodium sulfate, HFe: high-Fe diet (450 ppm), LSe: low-Se diet (0.02 ppm), MSe: medium-Se diet (0.1 ppm), HSe: high-Se diet (0.5 ppm). Data were represented as mean±SE. abMeans not sharing common superscript letters are significantly different at P<0.05. |
Changes in hematology
Table 2
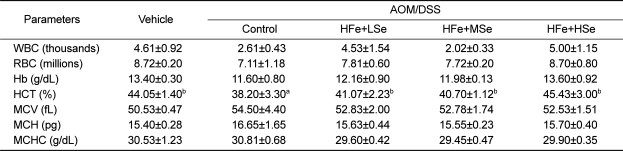
AOM: azoxymethane, DSS: dextran sodium sulfate, WBC: white blood cells, RBC: red blood cells, Hb: hemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, NFe: normal-Fe diet (45 ppm), HFe: high-Fe (450 ppm) diet, LSe: low-Se diet (0.02 ppm), MSe: medium-Se diet (0.1 ppm), HSe: high-Se diet (0.5 ppm).
Data represented as mean±SE. abMeans in each row with different superscripts are significantly different (P<0.05).
Fe concentration in the liver
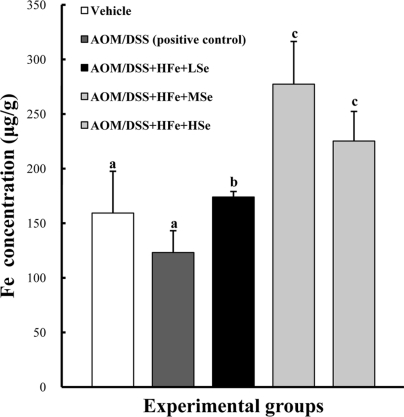 | Figure 2Hepatic Fe levels in mice fed the high Fe diet. Fe concentration was determined using an inductively coupled plasma spectrophotometer. High Fe diet significantly increased hepatic Fe levels compared with the control group. AOM: azoxymethane, DSS: dextran sodium sulfate, HFe: high-Fe diet (450 ppm), LSe: low-Se diet (0.02 ppm), MSe: medium-Se diet (0.1 ppm), HSe: high-Se diet (0.5 ppm). Data were represented as mean±SE. abcMeans not sharing common superscript letters are significantly different at P<0.05. |
Se concentration in the liver
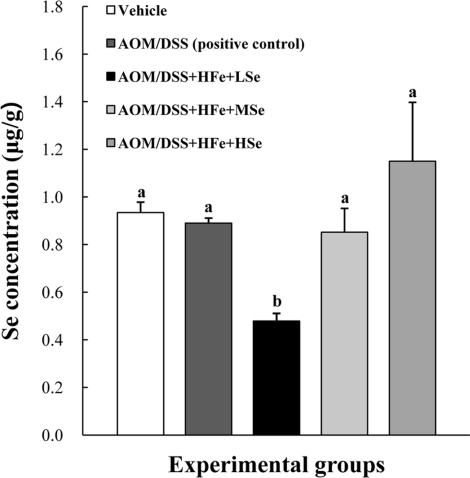 | Figure 3Hepatic Se levels in mice fed the high Fe diet with different Se levels. The Se concentration was determined using an inductively coupled plasma mass spectrophotometer. The hepatic Se levels were dependent on dietary Se levels. AOM: azoxymethane, DSS: dextran sodium sulfate, HFe: high-Fe diet (450 ppm), LSe: low-Se diet (0.02 ppm), MSe: medium-Se diet (0.1 ppm), HSe: high-Se diet (0.5 ppm). Data were represented as mean±SE. abMeans not sharing common superscript letters are significantly different at P<0.05. |
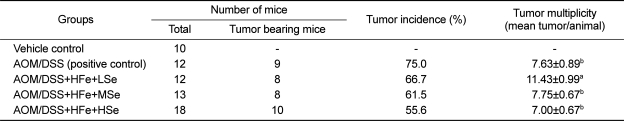
Tumor incidence and multiplicity
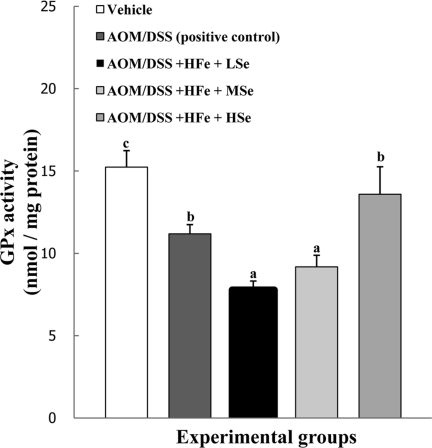
GPx activity in the liver
Expression levels of GPx-1 protein in the liver
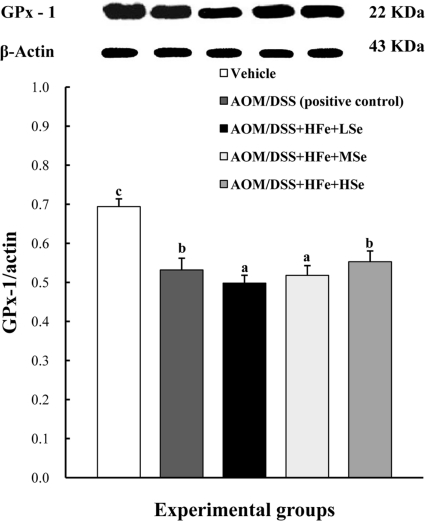 | Figure 5Effect of Se on hepatic GPx-1 by western blot in mice fed the high Fe diet with different Se levels. The expression of GPx-1 was up-regulated in the high-Se group compared with the low-Se group. AOM: azoxymethane, DSS: dextran sodium sulfate, HFe: high-Fe diet (450 ppm), LSe: low-Se diet (0.02 ppm), MSe: medium-Se diet (0.1 ppm), HSe: high-Se diet (0.5 ppm). Data were represented as mean±SE. abcMeans not sharing common superscript letters are significantly different at P<0.05. |
MDA levels in the liver
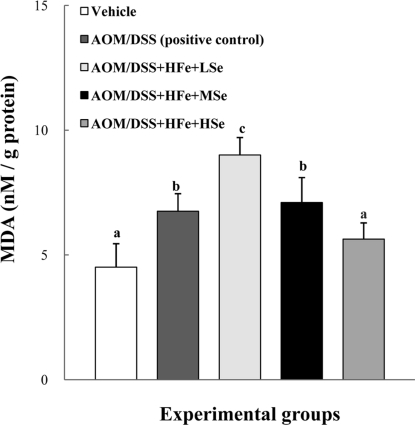 | Figure 6Effect of Se on hepatic MDA levels in mice fed the high Fe diet with different Se levels. The MDA levels were decreased by dietary Se in a dose-dependent manner. AOM: azoxymethane, DSS: dextran sodium sulfate, HFe: high-Fe diet (450 ppm), LSe: low-Se diet (0.02 ppm), MSe: medium-Se diet (0.1 ppm), HSe: high-Se diet (0.5 ppm). Data were represented as mean±SE. abcdMeans not sharing common superscript letters are significantly different at P<0.05. |
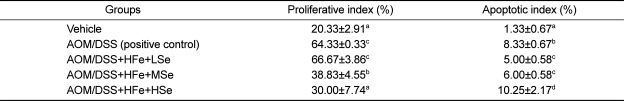
Effect of Se on cell proliferation and apoptosis in colon tumor
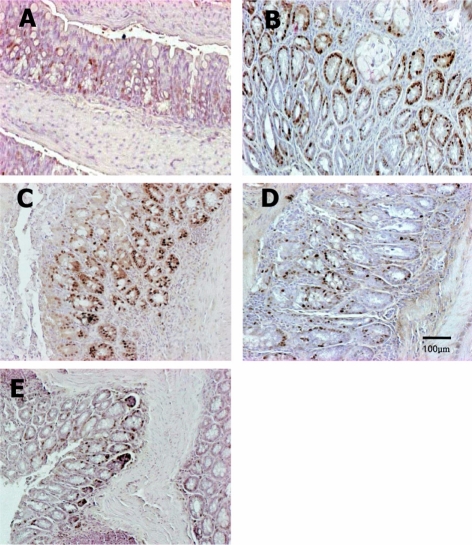 | Figure 7Immunohistochemistry of PCNA in the colon of mice fed the low Fe diet with different Se levels. The PCNA-positive cells were increased by treatment with AOM/DSS, but they were reduced by co-administration of a high concentration of Se. Vehicle (A), AOM/DSS+NFe+MSe (positive control, B), AOM/DSS+HFe+LSe (C), AOM/DSS+HFe+MSe (D), AOM/DSS+HFe+HSe (E). |
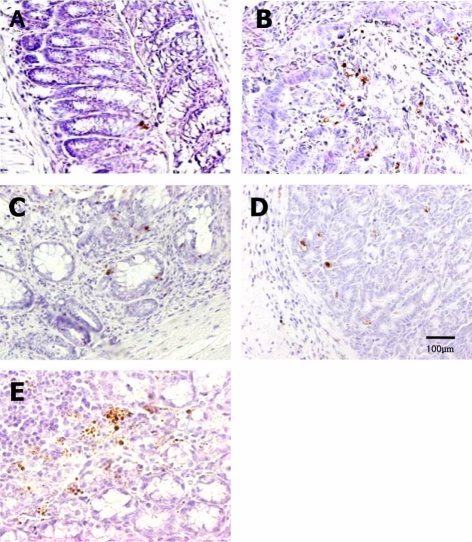 | Figure 8TUNEL assay for apoptotic nuclei in distal colon sections of mice fed high-Fe diet with different Se levels. The TUNEL-positive cells were increased by dietary Se in a dose-dependent manner. Vehicle (A), AOM/DSS+NFe+MSe (positive control, B), AOM/DSS+HFe+LSe (C), AOM/DSS+HFe+MSe (D), AOM/DSS+HFe+HSe (E). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download