Abstract
The extract of Alpinia katsumadai, a member of the family Zingiberaceae, shows anti-inflammatory effects and antioxidant activity. We observed the neuroprotective effects of the extract from Alpinia katsumadai seed (EAKS) against ischemic damage in gerbils administered oral EAKS (25, and 50 mg/kg) once a day for 7 days before transient cerebral ischemia. In the 50 mg/kg EAKS-treated ischemia group, about 67% of neurons in the hippocampal CA1 region (CA1) survived after ischemia/reperfusion (I/R) based on cresyl violet staining. We observed that EAKS treatment significantly maintained brain-derived neurotrophic factor (BDNF) immunoreactivity in the ischemic CA1 region after I/R. In addition, protein levels of BDNF in the 50 mg/kg EAKS-treated ischemia group were much higher than those in the vehicle-treated ischemia group after I/R. These findings indicate that repeated supplementation of EAKS protects neurons from ischemic damage, such that BDNF is distinctively maintained in ischemic areas.
Alpinia katsumadai is a member of the family Zingiberaceae that has been used for traditional Chinese medicine. This plant contains the following compounds: chalcone, flavonoids, diarylheptanoids, monoterpenes, sesquiterpenoids, stilbenes and labdanes (Saiki et al, 1978; Kuroyanagi et al, 1983; Yang et al, 1999). The extract of Alpinia katsumadai seed (EAKS) suppressed topical pruritis, showed anti-inflammatory effects, and enhanced antioxidant activity in several studies (Lee et al, 2003; Choi et al, 2009b). Hua et al (2009) also reported that EAKS has potent cytotoxic activity against the HepG2, MCF-7 and MAD-MB-435 cell lines.
Transient cerebral ischemia is known to result in neuronal death in some specific vulnerable regions such as the hippocampus, neocortex and striatum (Kirino, 1994). The Mongolian gerbil has been widely used as a model of transient cerebral ischemia, because the animal does not have a complete Willis' circle. Therefore, in this study, we investigated the neuroprotective effects of EAKS against delayed neuronal death in the hippocampal CA1 region using gerbils.
Alpinia katsumadai seeds were collected in Kangwon Province, Korea. The method of preparation of EAKS was reported previously by Hwang et al (2004). Briefly, for the preparation of ethanol EAKS, Alpinia katsumadai seeds were dried and ground into fine powder. The powder was dispersed in 75% ethanol and refluxed for 1 hour at 50℃. This extraction procedure was repeated three times. The ethanol extract was dried under vacuum.
Male Mongolian gerbils (6 months of age) were obtained from the Experimental Animal Center, Hallym University, Chuncheon, Korea. The procedures for handling animals and their care conformed to guidelines compliant with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996).
The animals were divided into the following groups: sham-operated gerbils (sham group), vehicle-treated ischemia gerbils (vehicle-ischemia group) and EAKS (25 and 50 mg/kg)-treated ischemia gerbils (EAKS-ischemia group). EAKS was orally administered through a feeding needle once a day for 7 days before transient ischemia, and the last treatment was 30 min before ischemia/reperfusion. Because in traditional medicine EAKS is taken orally and there are no data about the absorption and metabolism of EAKS (Yang et al, 2009), we selected oral administration of 50 mg/kg EAKS.
Gerbils underwent transient cerebral ischemia as in our previous study (Ahn et al, 2009). Briefly, the animals were anesthetized. Bilateral common carotid arteries were occluded for 5 minutes. The rectal temperature was monitored and maintained (37±0.5℃) before, during and after the surgery. Sham-operated animals were subjected to the same surgical procedures except that the common carotid arteries were not occluded.
To elucidate the protective effects of EAKS, brain sections from each group (n=7 in each group) were prepared at 4 days post-ischemia and stained with cresyl violet as we previously described. Cresyl violet-positive cells were counted using an image analyzing system (software: Optimas 6.5, CyberMetrics, Scottsdale, USA) (Choi et al, 2009a). We also examined the effect of brain-derived neurotrophic factor (BDNF) on ischemic damage at sham, 2 and 4 days post-ischemia (n=7 in each group) through immunochemistry using rabbit anti-BDNF (1:1,000; Chemicon International, Temecula, CA, USA) (Kim et al, 2007). In addition, we examined BDNF levels in the ischemic CA1 region of animals (n=5 in each group) through western blot analysis (Kim et al, 2007). The relative number of positive cells and the relative optical density of the bands of the Western blot analysis were shown as % of the sham group.
Data are expressed as the mean±SD. The data were evaluated by one-way ANOVA (SPSS program), and the means assessed using Duncan's multiple-range test. Statistical significance was considered at P<0.05.
In the sham group, neurons in the hippocampus were well stained with cresyl violet (Figures 1A and 1E). Delayed neuronal death in the hippocampal CA1 region was observed in the vehicle-ischemia group 4 days after ischemia/reperfusion (Figures 1B and 1F): About 13.8% of CA1 neurons were stained with cresyl violet (Figure 1I). In the 25 mg/kg EAKS-ischemia group, cresyl violet-positive cells were similar to those in the vehicle-ischemia group (Figures 1C, 1G and 1I). However, many neurons (about 67% of CA1 neurons) were well stained with cresyl violet in the 50 mg/kg EAKS-treated group (Figures 1D, 1H and 1I).
Change in BDNF immunoreactivity was well detected in the CA1 region of the vehicle-ischemia group (Figures 2A, 2C, 2E, 2G and 2I). In the vehicle-sham group, strong BDNF immunoreactivity was detected in the stratum pyramidale (Figure 1A). At 3 hours after ischemic insult, BDNF immunoreactivity was significantly decreased in the vehicle-ischemia group (Figure 2C). Thereafter, BDNF immuno-reactivity gradually decreased with time and was hardly detected at 4 days post-ischemia (Figures 2E, 2G and 2I). In each EAKS-ischemia group, BDNF immunoreactivity was significantly higher than in each vehicle-ischemia group (Figures 2D, 2F, 2H and 2J): the BDNF immunoreactivity was maintained in the stratum pyramidale of the CA1 region after 50 mg/kg EAKS treatment.
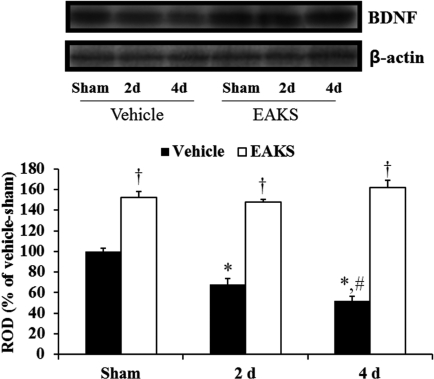
Western blot analysis also showed a similar change in BDNF levels in the vehicle-ischemia group compared to the immunohistochemical findings after ischemia/reperfusion (Figure 3): BDNF level in the vehicle-ischemia group was lowest at 4 days post-ischemia. In the 50 mg/kg EAKS-ischemia group, BDNF levels were higher than in the vehicle-ischemia group, showing levels that were similar to those in the EAKS-sham group.
Interestingly, extracts from natural substances have the ability to protect neurons from ischemic damage (Chun et al, 2008; Nazam Ansari et al, 2008; Yoo et al, 2010). The extracts have several functions including antioxidant effects in the neuroprotection from ischemic insults.
The administration of 50 mg/kg EAKS to gerbil protected the CA1 pyramidal neurons from ischemic damage: about 67% of the CA1 pyramidal neurons survived after transient ischemic insults. This is the first report to show that EAKS has neuroprotective effects in a gerbil model of ischemic damage. This finding is supported by a previous study in which EAKS inhibited H2O2-induced apoptosis and enhanced the viability of Chinese hamster lung fibroblasts (V79-4) (Lee et al, 2003).
The results of this study show that the EAKS-treated group had significantly increased BDNF immunoreactivity in the CA1 region after 12 hours post-ischemia compared to the vehicle-treated group. BDNF, a neurotrophic factor, has been associated with neuronal survival, synaptic plasticity, learning and memory, as well as neuronal plasticity (Miller and Kaplan, 2001; Ploughman et al, 2009). It has been reported that intraventricular treatment with BDNF reduced neuronal apoptosis induced by ischemia, suggesting that its effect is correlated with phosphorylation of Akt (Han and Holtzman, 2000). In addition, BDNF treatment prevented arabinoside-induced apoptosis in cultured cerebellar granular cells and glutamate-induced apoptosis in hippocampal neurons by increasing Akt and Bcl-2 levels (Almeida et al, 2005; Leeds et al, 2005).
In conclusion, the findings of the present study demonstrate that repeated oral administration of EAKS protects neurons from ischemic damage in the hippocampus in a gerbil model of transient cerebral ischemia, and indicate that the effect might be associated with an upregulation of BDNF, a neurotrophic factor.
Acknowledgments
The authors would like to thank Mr. Seung Uk Lee and Ms. Hyun Sook Kim for their technical help in this study. This research was supported by the Regional Core Research Program funded by the Korea Ministry of Education, Science and Technology (Medical & Bio-material Research Center).
References
1. Ahn HC, Yoo KY, Hwang IK, Cho JH, Lee CH, Choi JH, Li H, Cho BR, Kim YM, Won MH. Ischemia-related changes in naive and mutant forms of ubiquitin and neuroprotective effects of ubiquitin in the hippocampus following experimental transient ischemic damage. Exp Neurol. 2009; 220(1):120–132. PMID: 19666022.

2. Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005; 12(10):1329–1343. PMID: 15905876.

3. Choi JH, Yoo KY, Park OK, Lee CH, Won MH, Hwang IK, Ryu SY, Kim YS, Yi JS, Bae YS, Kang IJ. Platycodin D and 2"-O-acetyl-polygalacin D2 isolated from Platycodon grandiflorum protect ischemia/reperfusion injury in the gerbil hippocampus. Brain Res. 2009a; 1279:197–208. PMID: 19433075.
4. Choi JK, Kim KM, Kim DK, Yeom MH, Koh JY, Jung SJ, Kim HJ, Oh SH, Kim SY, Lee CH. Topical anti-inflammatory and antipruritic effects of Alpinia katsumadai extracts. J Dermatol Sci. 2009b; 53(1):81–84. PMID: 18771901.
5. Chun HS, Kim JM, Choi EH, Chang N. Neuroprotective effects of several Korean medicinal plants traditionally used for stroke remedy. J Med Food. 2008; 11(2):246–251. PMID: 18598165.

6. Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000; 20(15):5775–5781. PMID: 10908618.
7. Hua SZ, Luo JG, Wang XB, Wang JS, Kong LY. Two novel monoterpene-chalcone conjugates isolated from the seeds of Alpinia katsumadai. Bioorg Med Chem Lett. 2009; 19(10):2728–2730. PMID: 19362829.
8. Hwang IK, Yoo KY, Kim DS, Jeong YK, Kim JD, Shin HK, Lim SS, Yoo ID, Kang TC, Kim DW, Moon WK, Won MH. Neuroprotective effects of grape seed extract on neuronal injury by inhibiting DNA damage in the gerbil hippocampus after transient forebrain ischemia. Life Sci. 2004; 75(16):1989–2001. PMID: 15306166.

9. Kim DH, Li H, Yoo KY, Lee BH, Hwang IK, Won MH. Effects of fluoxetine on ischemic cells and expressions in BDNF and some antioxidants in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol. 2007; 204(2):748–758. PMID: 17328894.

10. Kirino T. Cerebral ischemia and neuronal death. No To Hattatsu. 1994; 26(2):130–135. PMID: 8136185.
11. Kuroyanagi M, Noro T, Fukushima S, Aiyama R, Ikuta A, Itokawa I, Morita M. Studies of constituents of seeds of Alpinia katsumadai Hayata. Chem Pharm Bull. 1983; 31(5):1544–1550.
12. Lee SE, Shin HT, Hwang HJ, Kim JH. Antioxidant activity of extracts from Alpinia katsumadai seed. Phytother Res. 2003; 17(9):1041–1047. PMID: 14595584.
13. Leeds P, Leng Y, Chalecka-Franaszek E, Chuang DM. Neurotrophins protect against cytosine arabinoside-induced apoptosis of immature rat cerebellar neurons. Neurochem Int. 2005; 46(1):61–72. PMID: 15567516.

14. Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001; 58(8):1045–1053. PMID: 11529497.

15. Nazam Ansari M, Bhandari U, Islam F, Tripathi CD. Evaluation of antioxidant and neuroprotective effect of ethanolic extract of Embelia ribes Burm in focal cerebral ischemia/reperfusion-induced oxidative stress in rats. Fundam Clin Pharmacol. 2008; 22(3):305–314. PMID: 18485149.
16. Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009; 40(4):1490–1495. PMID: 19164786.

17. Saiki Y, Ishikawa Y, Uchida M, Fukushima S. Essential oil from Chinese drug 'caodoukou', the seeds of Alpinia katsumadai. Phytochemistry. 1978; 17:808–809.
18. Yang J, Dai Y, Xia YF, Huang WZ, Wang ZT. Alpinia katsumadai Hayata prevents mouse sepsis induced by cecal ligation and puncture through promoting bacterial clearance and downregulating systemic inflammation. Phytother Res. 2009; 23(2):267–273. PMID: 18844287.
19. Yang Y, Kinoshita K, Koyama K, Takahashi K, Tai T, Nunoura Y, Watanabe K. Two novel anti-emetic principles of Alpinia katsumadai. J Nat Prod. 1999; 62(12):1672–1674. PMID: 10654416.
20. Yoo KY, Li H, Hwang IK, Choi JH, Lee CH, Kwon DY, Ryu SY, Kim YS, Kang IJ, Shin HC, Won MH. Zizyphus attenuates ischemic damage in the gerbil hippocampus via its antioxidant effect. J Med Food. 2010; 13(3):557–563. PMID: 20521981.

Figure 1
Cresyl violet staining in the hippocampus of sham (A, E), vehicle-ischemia (B, F), 25 mg EAKS-ischemia (C, G) and 50 mg EAKS-ischemia (D, H) group at 4 days post-ischemia. In the vehicle-ischemia and 25 mg EKAS-ischemia groups, only a few neurons in CA1 region (asterisk) are stained; in the 50 mg EAKS-ischemia group, many cresyl violet-positive cells are found. CA1, cornu ammonis 1; CA2/3, cornu ammonis 2/3; DG, dentate gyrus; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Scale bar=400 µm (A, B, C and D), 50 ìm (E, F, G and H). I: Relative number of cresyl violet-positive neurons in each group (n=7 per group; *P<0.05, significantly different from the sham group). Bars indicate mean±SD.
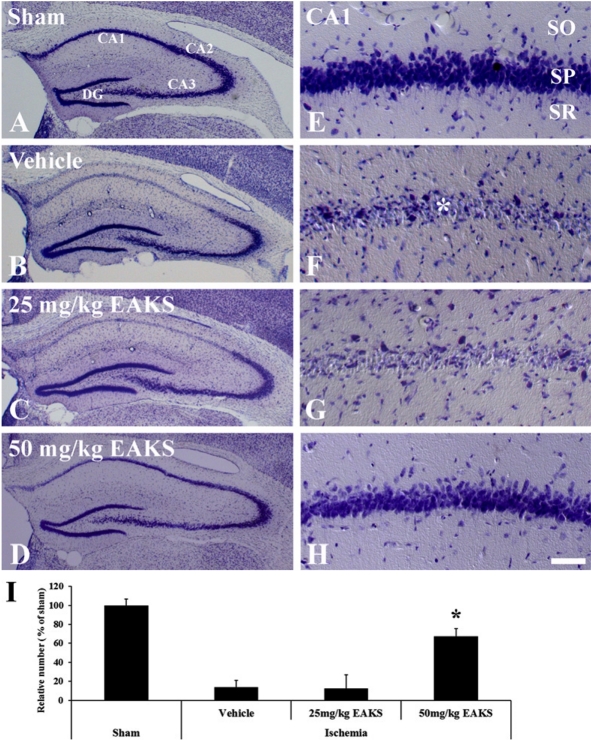
Figure 2
Immunohistochemistry for BDNF in the CA1 region in the sham (A, B), vehicle-ischemia and 50 mg EAKS-ischemia groups at 3 hours (C, D), 12 hours (E, F), 2 days (G, H) and 4 days (I, J) post-ischemia. BDNF immunoreactivity in the stratum pyramidale (SP) is gradually decreased with time; however, in the EAKS-ischemia group, BDNF immunoreactivity (asterisk) is maintained in pyramidal cells. SO, stratum oriens; SR, stratum radiatum. Scale bar=50 µm.
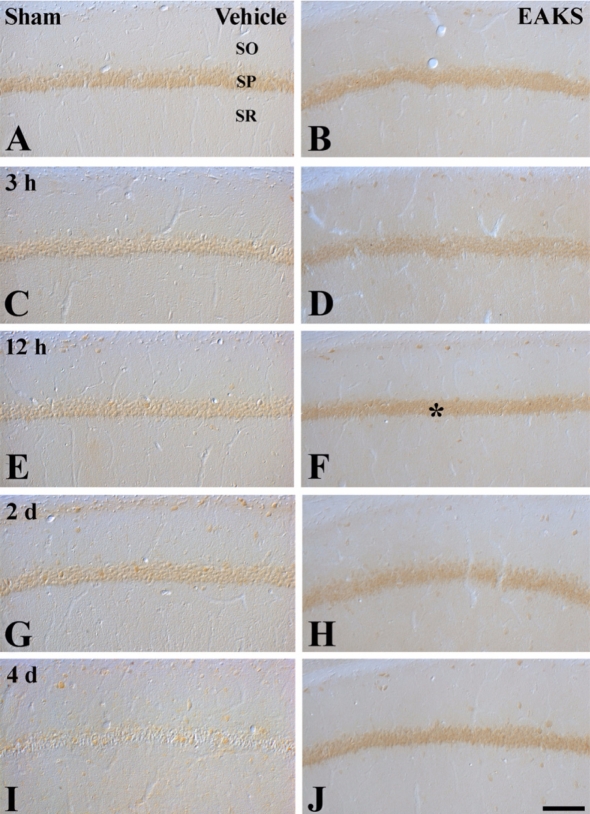
Figure 3
Western blot analysis of BDNF in the CA1 region of the sham, vehicle-ischemia and 50 mg EAKS-ischemia groups. Relative optical density (ROD) as a percentage of the immunoblot band is presented (n=5 per group; *P<0.05, significantly different from the sham group; #P<0.05, significantly different from the preceding group; †P<0.05, significantly different from the vehicle-ischemia group). Bars indicate mean±SD.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download