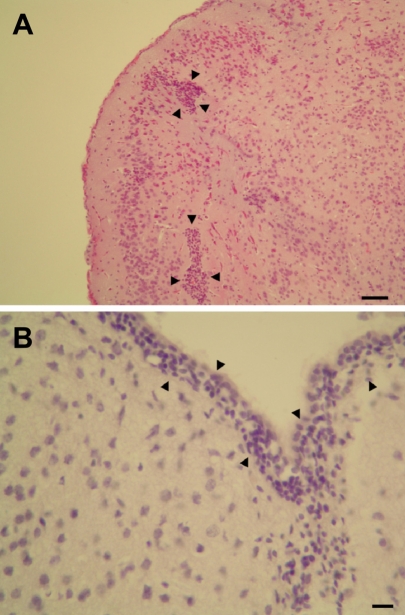
 | Figure 1Histopathological findings of the cerebrum of Cronobacter sakazakii-infected mice. A: gliosis (black arrows), bar=100 µm. B: meningitis (black arrows), bar=50 µm. Hematoxylin-eosin stain. |
Abstract
Cronobacter sakazakii (C. sakazakii), formerly Enterobacter sakazakii, is an emerging pathogen associated with the ingestion of contaminated reconstituted formula that causes serious illnesses such as bacteremia, septicemia, necrotizing enterocolitis, meningitis and death in low-birth-weight preterm neonatal infants. The objective of this study was to develop an animal model for human neonatal C. sakazakii infections. We acquired timed-pregnant ICR mice and allowed them to give birth naturally. On postnatal day 3.5, each pup was administered orally a total dose of approximately 107 CFU C. sakazakii strain 3439. Mice were observed twice daily for morbidity and mortality. At postnatal day 10.5, the remaining pups were euthanized, and brain, liver, and cecum were excised and analyzed for the presence of C. sakazakii. C. sakazakii was isolated from cecum and other tissues in inoculated mice. In the tissues of C. sakazakii infected mice, meningitis and gliosis were detected in brain. In this study, we confirmed the neonatal ICR mice may be used a very effective animal model for human neonatal C. sakazakii infections.
Go to : 
Neonatal infections believed to have been caused by Cronobacter sakazakii (C. sakazakii), formerly Enterobacter sakazakii (Iversen et al, 2008), were first reported by Urmenyi and Franklin (1961). Cronobacter are motile peritrichous, Gram-negative, rod-shaped, non-spore-forming bacteria (Iversen et al, 2004). Cronobacter is recognized worldwide as an emerging foodborne pathogen (Drudy et al, 2006). Cronobacter is distributed and frequently contaminated in the environment, plant materials, powdered infant formulas, cereal foods, fermented beverages, fruits, and vegetables (Drudy et al, 2006). In particular, contamination on powdered infant formula occurs more easily because it is a nonsterilized product (Beuchat et al, 2009). C. sakazakii infections are an important cause of life-threatening meningitis, septicemia, and necrotizing enterocolitis in infants and neonates (Nazarowec-White and Farber, 1997; Lai, 2001; Drudy et al, 2006). Premature and low-birth-weight infants and those aged <28 days are considered to be more at risk than are older infants (Bar-Oz et al, 2001). To examine C. sakazakii infection, human studies are unethical because mortality is a possible outcome for suitable individuals (Richardson et al, 2009). Animal surrogate studies are essential for extrapolation of C. sakazakii infection in humans. The design of an animal model for C. sakazakii infection is fundamental in gaining knowledge of why premature and immunocompromised human infants are at greater risk of infection. Furthermore, an animal model will allow us to test different strains of C. sakazakii for virulence (Richardson et al, 2009). The objective of this study was to evaluate the usability of neonatal ICR mice as an animal model for human C. sakazakii infections.
We acquired specific pathogen-free (SPF) timed-pregnant ICR mice at gestation day 15 from Samtako (Osan, Korea). Dams were acclimatized and kept in an isolated SPF barrier room with regulated temperature (23±1℃), humidity (50±5%) and light/dark cycle (12/12 hours). The animals were fed sterilized pellet diet by 2 M rad radiation (Purina, Seongnam, Korea) and sterilized water ad libitum. Dams were allowed to give birth naturally. Litters averaged 11 pups were divided with 2 groups (infection and control) and kept in an opaque, polypropylene cage under a small animal isolator. Neonates were treated orally by gavage on postnatal day 3.5 by using a 24 gauge, 3.175 stainless steel animal feeding tube (Popper & Sons, New York, USA) attached to a 1 mL syringe. In the other study, the oral infectious dose has been approximated to range from 103 (Iversen and Forsythe, 2003) to ≥108 CFU (Pagotto et al, 2003). In this study, 3.5 day-old mice pups (n=11) inoculated orally with 107 CFU of C. sakazakii strain 3439 (isolated from commercially manufactured powdered infant formula) in 0.1 mL of hydrated infant formula with feeding tube. Control mice (n=11) received saline through the same route. Mice were observed twice daily for morbidity and mortality. We defined morbidity as noticeable lethargy and change of skin color from pink to blue or grey. At postnatal day 10.5, the remaining pups were euthanized, and brain, liver, small intestine and cecum were excised and cultured for C. sakazakii from the neonates. Also, the tissues were submitted to histopathological analysis. All studies were performed in accordance with the Guide for Animal Experimentation by Wonkwang University and approved by the Institutional Animal Care and Use Committee of Wonkwang University. All efforts were made to minimize pain or discomfort of animals used.
Each organ was aseptically taken from each mouse, mixed with 100 mL of Enterobacteriaceae enrichment (EE) broth (BD/Difco, Sparks, USA), and pummeled in a stomacher (Seward Medical, London, UK) at 260 rpm for 1 min. The mixtures were incubated at 37℃ overnight for enrichment of C. sakazakii in tissues. After incubation, the EE broth was streaked onto violet red bile glucose (VRBG) agar (BD/Difco) and incubated at 37℃ for 16-18 hours. Cells from presumptive C. sakazakii colonies were streaked on tryptic soy agar (BD/Difco) and incubated at 25℃ for 16-18 hours. Cells from yellow-pigmented colonies were subjected to confirmation using the API 20E kit. Simultaneously, 16s rRNA gene sequencing of cells from presumptive C. sakazakii colonies was performed by Macrogen (Seoul, Korea; http://www.macrogen.com). The 16s rRNA gene sequences were analyzed by an NCBI BLAST search (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) for identification of C. sakazakii.
For histopatholocal analysis, the exercised tissues were fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. Then, 4 µm sections were cut on microtome (Thermo Shandon, Cheshire, UK) and stained with hematoxylin and eosin.
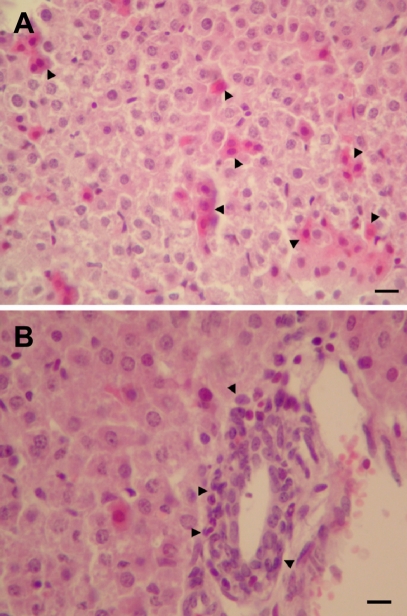
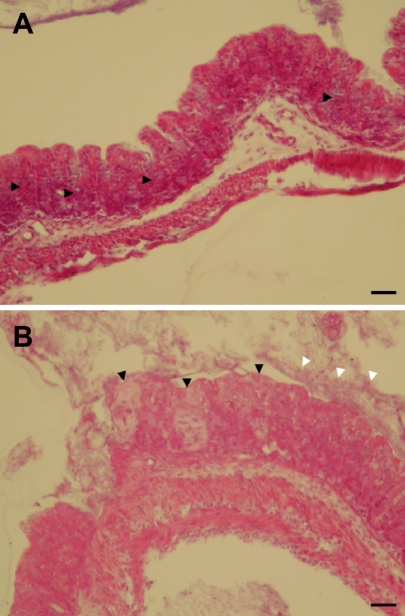
In control mice, there were no dead animals during the experiment period. However, in mice of the infection group, 6 cases were dead at 3 days post-inoculation of C. sakazakii. Seven days after treating 3.5-day-old neonatal mice by oral gavage, C. sakazakii isolated from liver (3 cases in 5 survival pups), brain (2 cases in 5 survival pups), and cecum (5 cases in 5 survival pups) of C. sakazakii infected mice. In control mice, there were no histopathological changes. However, histopathological findings of the C. sakazakii-infected mice were meningitis and gliosis, and inflammatory cell infiltration in the cerebrum (3 cases in 5 survival pups) (Figure 1), and inflammatory cell infiltration and cellular degeneration in the liver (3 cases in 5 survival pups) (Figure 2), and inflammatory cell infiltration and cellular degeneration in the cecum (5 cases in 5 survival pups) (Figure 3).
C. sakazakii has been isolated from a wide range of environmental sources and foods of animal and plant origins, however, outbreaks of its infections have been linked only to powdered infant formula (Beuchat et al, 2009). Developing animal model as a surrogate for C. sakazakii infection in premature infants is an important step forward, enabling subsequent research into understanding the mechanisms of infection, morbidity, prevention and treatment (Richardson et al, 2009). C. sakazakii is an opportunistic pathogen causing invasive infections (meningitis, sepsis, and necrotizing enterocolitis) with high death rates (40-80%), primarily in newborns (Bar-Oz et al, 2001; Iversen and Forsythe, 2003). In this study, we identified neonatal ICR mice as an animal model of C. sakazakii infection in premature infants. We observed C. sakazakii infection-related deaths in infected neonatal mice. Also, C. sakazakii isolated from liver, brain, and cecum of infected survival mice. In the C. sakazakii-infected mice, histopathological changes were detected in brain, liver and cecum. Our results indicate that C. sakazakii could be colonized and replication in ICR mice. Our results also show that C. sakazakii can induce meningitis and gliosis in neonatal mice. This is important because meningitis and other neurological squels are known to occur in human infants because of C. sakazakii infection. Mice are the most commonly used vertebrate species, popular because of their availability, size, low cost, ease of handling, and fast reproduction rate (Zak, 1999). ICR mice are the most popular strain of mice (Willis-Owen and Flint, 2006). In this study, we confirmed the neonatal ICR mice may be used a very effective animal model for human neonatal C. sakazakii infections. In conclusion, these results demonstrate that the neonatal ICR mice model may be used a very effective animal model for human neonatal C. sakazakii infections.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021940).
Go to : 
References
1. Bar-Oz B, Preminger A, Peleg O, Block C, Arad I. Enterobacter sakazakii infection in the newborn. Acta Paediatr. 2001; 90(3):356–358. PMID: 11332182.
2. Beuchat LR, Kim H, Gurtler JB, Lin LC, Ryu JH, Richards GM. Cronobacter sakazakii in foods and factors affecting its survival, growth, and inactivation. Int J Food Microbiol. 2009; 136(2):204–213. PMID: 19346021.
3. Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin Infect Dis. 2006; 42(7):996–1002. PMID: 16511766.
4. Iversen C, Forsythe SJ. Risk profile of Enterobacter sakazakii, an emergent pathogen associated with infant milk formula. Trends Food Sci Technol. 2003; 14(11):443–454.
5. Iversen C, Mullane N, McCardel B, Tal BD, Lehner A, Fannin S, Stephan R, Joosten H. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, and proposal of Cronobacter sakazakii gen. nov., comb. nov., Cronobacter malonaticus sp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov., Cronobacter genomospecies 1, and of three subspecies, Cronobacter dublinensis subsp. dublinensis subsp. nov., Cronobacter dublinensis subsp. lausannensis subsp. nov. and Cronobacter dublinensis subsp. lactaridi subsp. nov. Int J Syst Evol Microbiol. 2008; 58(6):1442–1447. PMID: 18523192.
6. Iversen C, Waddington M, On SL, Forsythe S. Identification and phylogeny of Enterobacter sakazakii relative to Enterobacter and Citrobacter species. J Clin Microbiol. 2004; 42(11):5368–5370. PMID: 15528745.
7. Lai KK. Enterobacter sakazakii infections among neonates, infants, children, and adults: case reports and a review of the literature. Medicine (Baltimore). 2001; 80(2):113–122. PMID: 11307587.
8. Nazarowec-White M, Farber JM. Enterobacter sakazakii: a review. Int J Food Microbiol. 1997; 34(2):103–113. PMID: 9039558.
9. Pagotto FJ, Nazarowec-White M, Bidawid S, Farber JM. Enterobacter sakazakii: infectivity and enterotoxin production in vitro and on vivo. J Food Prot. 2003; 66(3):370–377. PMID: 12636287.
10. Richardson AN, Lambert S, Smith MA. Neonatal mice as models for Cronobacter sakazakii infection in infants. J Food Prot. 2009; 72(11):2363–2367. PMID: 19903401.
11. Urmenyi AMC, Franklin AW. Neonatal death from pigmented coliform infection. Lancet. 1961; 1(7172):313–315. PMID: 13779326.

12. Willis-Owen SA, Flint J. The genetic basis of emotional behaviour in mice. Eur J Hum Genet. 2006; 14(6):721–728. PMID: 16721408.

13. Zak O. Zak O, editor. Introductory background to animal models of infection. Handbook of Animal Models and Infection: Experimental Models in Antimicrobial Chemotherapy. 1999. 1st ed. San Diego: Academic Press;p. 1–117.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download