Abstract
Antiulcer effects of pantoprazole, a proton-pump inhibitor, on water-immersion restraint stress (WIRS)-, alcohol (ethanol)- and pylorus ligation-induced gastric ulcers were investigated in male rats. Rats were orally administered with pantoprazole 30 min prior to exposure to various types of ulcer inducers. In stress-induced ulcer model, rats were subjected to WIRS at 22℃ for 4 hours, and the degree of ulcer (in mm) was evaluated. In alcohol-induced ulcer model, rats were orally administered with pure (100%) ethanol (1 mL/kg), and the ulcer lesions were measured 1 hour after ethanol challenge. In pylorus ligation-induced ulcer model, rats were subjected to pylorus ligation, and the degree of erosions and ulcers was scored 17 hours after the operation. Pantoprazole attenuated the ulcer lesions induced by WIRS in a dose-dependent manner, exhibiting a median effective dose (ED50) value of 0.78 mg/kg. By comparison, pantoprazole was effective at relatively-high doses for the improvement of ethanol-induced ulcers, showing an ED50 value of 20.5 mg/kg. Notably, pantoprazole was practically ineffective (ED50>50.0) in pylorus ligation model. Taken together, it was confirmed that pantoprazole showed inhibitory activity on gastric ulcers induced by stress and alcohol, but was ineffective on pylorus ligation-induced ulcer. Therefore, the results indicate that proton-pump inhibitors including pantoprazole might reveal highly-different effects according to the type of ulcer inducers, and that the prescription of antiulcer agents should be carefully selected.
Peptic ulcers are injury of the mucosal area immersed in gastric acid and pepsin, wherein the area is covered normally with mucin secreted from mucus cells (Wallace and Granger, 1996; Neal, 2003). Ulceration results from the imbalance between gastroprotective and aggressive factors (Lou et al, 2006). The aggressive factors such as increased gastric secretion and retention, increased generation of reactive oxygen species (ROS) and proinflammatory cytokines such as interleukin-1β (IL-1β), tumor-necrosis factor-α (TNF-α) and IL-6, and blood flow disturbances cause mucosal damages (Hemmer et al, 1980; Konturek et al, 1990; Das and Banerjee, 1993). An apoptosis is also causative factor for gastric ulceration (Piotrowski et al, 1997a, 1997b; Fujii et al, 2000). An imbalance between the Bcl-2 family of antiapoptotic protein and apoptotic Bax protein in the early stage of gastric ulcer promotes apoptosis (Slomiany et al, 1997; Fujii et al, 2000).
Gastric ulcer is a serious injury occurring by spicy food, stress, alcohol, gastric surgery and Helicobacter (H.) pylori (Neal, 2003). In many studies, water-immersion restraint stress (WIRS)-, alcohol (ethanol)- and pylorus ligation-induced gastric ulcer models have been used to screen antiulcer compounds, since mechanisms of those models are different (Kitagawa et al, 1979; Wallace and Granger, 1996; Shah et al, 2003; Isobe et al, 2004; Cao et al, 2005; Byun et al, 2007; Raffin et al, 2007). WIRS, a generalized model to induce extreme mental and psychological stresses to rodents, stimulates gastric secretion related to central nervous system as well as pituitary-renal hormonal axis (Kitagawa et al, 1979; Wallace and Granger, 1996; Neal, 2003; Isobe et al, 2004; Cao et al, 2005; Byun et al, 2007). Ethanol causes not only mucosal stasis, leading to hemorrhage and necrosis, but also lipid peroxidation by triggering radical reactions (Shah et al, 2003; Raffin et al, 2007). Long-term pylorus ligation induces retention of gastric acid secreted by cholinergic activation and stimulates activity of pepsin, resulting in gastric wall injury (Wallace and Granger, 1996; Neal, 2003).
The enzyme H+/K+-ATPase pumps protons in exchange for potassium ions across the apical membrane to secrete gastric acid by parietal cells (Wallmark et al, 1985). Histamine antagonists including cimetidine and ranitidine as well as proton pump inhibitors such as omeprazole and lansoprazole are well known effective drugs for the treatment of peptic ulcers by decreasing the gastric secretion (Neal, 2003). Pantoprazole, a water-soluble benzimidazole, is converted to its active form inside the gastric parietal cells, also improves acid-related, hypersecretory gastrointestinal disorders by irreversibly inhibiting H+/K+-ATPase activity (Lindberg et al, 1986; Fitton et al, 1996; Jungnickel, 2000; Raffin et al, 2007). In addition, pantoprazole has a cytoprotective activity, which might attenuate the gastric mucosal injury (Lou et al, 2006; Raffin et al, 2007).
It was believed that pantoprazole, as a broad spectrum antiulcer agent, exerts improving effects on various types of gastric ulcer. However, it was assumed that pantoprazole possesses different therapeutic ranges according to the inducers of gastric mucosal injury. Therefore, this study was performed to investigate the comparative antiulcer activities of pantoprazole in WIRS-, alcohol- and pylorus ligation-induced ulcer models.
Six-week-old male Sprague-Dawley rats (n=10 per group) weighting 200-220 g were purchased from the Orient Bio Inc. (Gapyeong, Gyeonggi, Korea). The animals were housed in each cage with free access to feed and water under constant environmental conditions (22±2℃; 40-70% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). All the animal experiments were adhered to the Standard Operation Procedures and approved by the Institutional Animal Care and Use Committee of Chungbuk National University.
Rats were fasted 48 hours with only free access to water before performance of the experiment. Each rat was orally administered 6 dose levels (0.625, 1.25, 1.875, 2.5, 5 or 10 mg/kg) of pantoprazole (Sigma-Aldrich; St. Louis, MO, USA) or its vehicle [1% carboxymethylcellulose (CMC), 5 mL/kg]. Thirty min later, the rats were placed in a restraint device and immersed up to their xiphoid process in a 22℃ water bath for 4 hours for the induction of gastric ulcer (Byun et al, 2007). After sacrifice of the animals, their stomachs were removed, inflated with 1% formalin (10 mL), and fixed in the formalin solution for 15 min. The stomachs were longitudinally excised along the great curvature. The gastric content was removed by gentle wiping with Kimwipes, and the stomach was unwrapped and fixed on a corkboard with pins. The length of ulcer (hemorrhagic) lesions were measured, in which 5 petechial lesions was calculated as 1 mm. Ulcer index was presented as the total length (mm) of the lesions. The results were expressed as a median effective dose (ED50) based on the dose-dependent inhibition rates.
After 48-hour fasting, animals were orally administered with 4 dose levels (5, 10, 25 or 50 mg/kg) of pantoprazole or its vehicle (CMC). Thirty min later, the rats were orally administered with 1 mL/kg of ethanol for the induction of gastric ulcer (Kim et al, 2008). The animals were sacrificed 1 hour after ethanol administration, and their stomachs were removed. The stomachs were processed and examined as described above.
After 48-hour fasting, animals were orally administered with 5 dose levels (2.5, 5, 10, 25 or 50 mg/kg) of pantoprazole or its vehicle (CMC). Thirty min later, the rats were subjected to an operation which was approached from abdominal median incision. The pylorus connected to duodenum was ligated, and the peritoneum and skin were sutured (Shay et al, 1945; Cao et al, 2005). The animals were sacrificed 17 hours after pylorus ligation, and their stomachs were removed. The stomachs were processed as described above, and the gastric mucosal lesions were examined under a steromicroscope (×3). The number and degree of erosions and ulcers were scored in 0-5 levels as described in the Table 1 (Cantarella et al, 2005, 2007). The results were expressed as ED50 based on the dose-dependent inhibition rates.
Gastric erosions and ulcers are induced by various factors including gastric oversecretion and retention, weakening and depleting agents of mucin layer, blood flow disturbances, and mucosal injury and inflammation (Wallace and Granger, 1996; Neal, 2003; Isobe et al, 2004; Byun et al, 2007). The ulcer-inducing agents include non-steroidal antiinflammatory drugs (NSAID) that block production of prostaglandins, leading to mucin depletion and blood flow disturbances (Slomiany et al, 1997; Filaretova et al, 2002; Cao et al, 2004; Rao et al, 2004; Kim et al, 2005), alcohols (Cao et al, 2004; Rao et al, 2004; Raffin et al, 2007), stresses (Cao et al, 2004; Rao et al, 2004; Byun et al, 2007), gastric oversecretion and retention (Rao et al, 2004; Cao et al, 2005), gastric hypermotility and acetic acid accumulation (Dias et al, 2000; Rao et al, 2004; Cantarella et al, 2005; Isbil et al, 2006; Cantarella et al, 2007), and H. pylori infection (Wallace and Granger, 1996; Neal, 2003). For the therapy of gastric ulcers, proton-pump inhibitors that block acid secretion from parietal cells, antacids, histamine receptor (H2) antagonists, prostaglandins that strengthen mucin layer, and antibiotics to eliminate H. pylori have been used (Wallace and Granger, 1996; Neal, 2003).
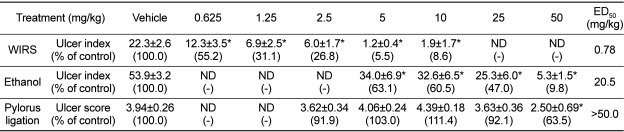
In the present study, the antiulcer effects of pantoprazole, a proton-pump inhibitor, was investigated in WIRS-, ethanol- and pylorus ligation-induced gastric ulcer models, and the comparative efficacies of pantoprazole were evaluated. Four-hour WIRS produced a linear type of hemorrhagic ulcers (Figure 1A), showing mean ulcer index of 22.3 mm. Such lesions were attenuated by pretreatment with pantoprazole in a dose-dependent manner (Figure 1B). The ulcer index was inhibited to 55.2, 31.1, 26.8, 5.5, and 8.6% of control value by 0.625, 1.25, 2.5, 5 and 10 mg/kg of pantoprazole, respectively, leading to 0.78 mg/kg of ED50 (Table 2). Such an excellent effect of pantoprazole proton-pump inhibitor was expected from the previous results that gastric secretion was facilitated during severe stresses (Kitagawa et al, 1979; Wallace and Granger, 1996; Neal, 2003; Cao et al, 2004). Furthermore, proton-pump inhibitors including pantoprazole and lansoprazole prevent mucosal cells from cytotoxicity and death induced by indomethacin, stress and ethanol, suggesting that their cytoprotective effect is also an additional key factor for the antiulcer activity (Cao et al, 2004; Lou et al, 2006; Raffin et al, 2007).
Oral administration of ethanol (1 mL/kg) induced many linear hemorrhagic ulcers 1 hour after administration (Figure 2A), showing mean ulcer index of 53.9 mm. The lesions were attenuated by pretreatment with pantoprazole in a dose-dependent manner (Figure 2B). The ulcer index was inhibited to 63.1, 60.5, 47.0, and 9.8% of control value by 5, 10, 25 and 50 mg/kg of pantoprazole, respectively (Table 2). However, the effective dose was much higher than in stress-induced ulcer model, showing 20.5 mg/kg of ED50. Ethanol was reported to facilitate gastric secretion, which may contribute to some degree to ulceration. Histamine receptor 2 (H2)-mediated gastric secretion is not involved in the alcoholic ulcer, since the ethanol-induced lesions are not suppressed by cimetidine (Robert et al, 1979; Raffin et al, 2007). Instead, it was proposed that mucosal stasis and radical reactions are the main stream cascade for the induction of alcoholic mucosal injury (Shah et al, 2003; Raffin et al, 2007). Therefore, it is suggested that the effect of pantoprazole comes from the cytoprotective activity, not related to prostaglandins synthesis (Konturek et al, 1983; Cao et al, 2004). However, the overall effectiveness of pantoprazole was practically low for the alcohol-induced ulcer as shown in the present study.
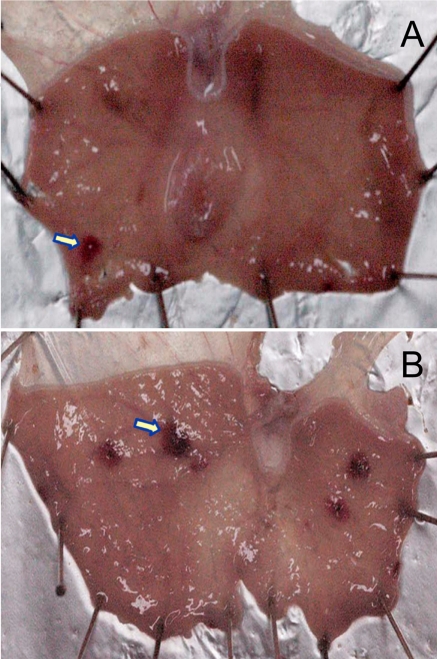
Seventeen-hour pylorus ligation caused focal erosions and petechial ulcers (Figure 3A), showing mean score of 3.94 (maximum score 5.0). Although the lesions were attenuated by 30-min pretreatment with pantoprazole, the effects were obtained by only very high doses without dose-dependent responses at low doses (5-10 mg/kg) (Figure 3B), resulting in an ED50 of over 50.0 mg/kg (Table 2). In the present study, pantoprazole exhibited biphasic activities, which may be due to its suppression of gastric secretion and direct cytoprotection at low and high doses, respectively (Cao et al, 2004, 2005). Notably, pylorus ligation induced mucosal and submucosal hemorrhages, rather than erosions and ulcers (Figure 3), making investigators difficult to measure the length or area of the ulcer lesions. In this context, we adopted a scoring system which has been used in reserpine model (Cantarella et al, 2005, 2007), to evaluate more accurately diverse types of the lesions including hemorrhages (petechia and ecchymosis), erosions and ulcers. It is of interest to note that there was a big difference in the effective doses between a previous report (Cao et al, 2005) and our present observations. Although the measuring systems were different between the 2 studies (ulcer area versus lesion score), the discrepancy remains to be clarified.
In spite of multiple mechanisms, gastric secretion is one of the main factors for ulceration, because most types of the ulcers induced by diverse agents were attenuated by the secretion inhibitors (Cao et al, 2004). Activated pantoprazole in parietal cells reduces gastric secretion by irreversibly inhibiting the proton-pump H+/K+-ATPase (Kromer et al, 1990; Cao et al, 2004; Raffin et al, 2007). In addition to gastric secretion, however, several factors such as cell death and recovery, inflammation, stasis and blood flow are involved in the process of ulcer formation (Lou et al, 2006). Especially, the effectiveness of pantoprazole was poor in pylorus ligation model. Thus, additional investigations on the effects of pantoprazole using various models remain to be clarified.
In the present study, it was demonstrated that pantoprazole has different improving effects in WIRS-, ethanol- and pylorus ligation-induced gastric ulcer models: i.e., highly effective, practically low effective, and ineffective, respectively. Therefore, it is suggested that the effects of proton-pump inhibitors including pantoprazole might be highly different according to the type of ulcer inducers, and that the prescription of antiulcer agents should be carefully selected.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094035).
References
1. Byun SK, Lee YE, Shin SH, Jang JY, Choi BI, Park DS, Jeon JH, Nahm SS, Hwang SY, Kim YB. The role of corticosteroids in stress-induced gastric ulceration in rats. Lab Anim Res. 2007; 23:127–131.
2. Cantarella G, Martinez G, Cutuli VM, Loreto C, D'Alcamo M, Prato A, Amico-Roxas M, Bernardini R, Clementi G. Adrenomedullin modulates COX-2 and HGF expression in reserpine-injured gastric mucosa in the rat. Eur J Pharmacol. 2005; 518:221–226. PMID: 16081063.
3. Cantarella G, Martinez G, Di Benedetto G, Loreto C, Musumeci G, Prato A, Lempereur L, Matera M, Amico-Roxas M, Bernardini R, Clementi G. Protective effects of amylin on reserpine-injured gastric damage in the rat. Pharmacol Res. 2007; 56:27–34. PMID: 17412609.
4. Cao H, Wang MW, Jia JH, Wang QG, Cheng MS. Comparison of the effects of pantoprazole enantimers on gastric mucosal lesions and gastric epithelial cells in rats. J Health Sci. 2004; 50:1–8.
5. Cao H, Wang MW, Sun LX, Ikejima T, Hu ZQ, Zhao WH. Pharmacodynamic comparison of pantoprazole enantimers: inhibition of acid-related lesions and acid secretion in rats and guinea-pigs. J Pharm Pharmacol. 2005; 57:923–927. PMID: 15969954.
6. Das D, Banerjee RK. Effect of stress on the antioxidant enzymesand gastric ulceration. Mol Cell Biochem. 1993; 125(2):115–125. PMID: 8283967.
7. Dias PC, Foglio MA, Possenti A, de Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethnopharmacol. 2000; 69:57–62. PMID: 10661884.
8. Filaretova L, Tanaka A, Miyazawa T, Kato S, Takeuchi K. Mechanisms by which endogenous glucocorticoid protects against indomethacin-induced gastric injury in rats. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G1082–G1089. PMID: 12381521.
9. Fitton A, Wiseman L. Pantoprazole: a review of its pharmacological properties and therapeutic use in acid-related disorders. Drugs. 1996; 51:460–482. PMID: 8882382.
10. Fujii Y, Matsura T, Kai M, Matsui H, Kawasaki H, Yamada K. Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells. Proc Soc Exp Biol Med. 2000; 224(2):102–108. PMID: 10806417.

11. Hemmer J, Schwille PO, Schellerer W, Hofmann W. Effects of cimetidine upon gastric secretion and mucosal blood flow in the rat stressed by restraint. A dose-response and prophylaxis trial. Res Exp Med. 1980; 176(3):207–217.
12. Işbil Büyükcoşkun N, Güleç G, Ozlük K. Protective effect of centrally-injected glucagons-like peptide-1 on reserpine-induced gastric mucosal lesions in rat: possible mechanisms. Turk J Gastroenterol. 2006; 17:1–6. PMID: 16830270.
13. Isobe H, Okajima K, Harada N, Liu W, Okabe H. Activated protein C reduces stress-induced gastric mucosal injury in rats by inhibiting the endothelial cell injury. J Thromb Haemost. 2004; 2:313–320. PMID: 14995995.

14. Jungnickel PW. Pantoprazole: a new proton pump inhibitor. Clin Ther. 2000; 22:1268–1293. PMID: 11117653.

15. Kim YR, Lee MR, Kim YH, Jang BJ, Park SC, Han SH, Kim BH, Ryoo ZY, Kim KS. Effect of Opuntiahumifusa extract on indomethacin-induced gastric ulcer in Sprague Dawley rat. Lab Anim Res. 2005; 21:375–578.
16. Kim JJ, Kim CH, Park D, Shin S, Jeon JH, Jang MJ, Ji HJ, O N, Song J, Lee J, Kim B-Y, Choi EK, Joo SS, Hwang S-Y, Kim YB. Effects of sweet potato fractions on alcoholic hangover and gastric ulcer. Lab Anim Res. 2008; 24:209–216.
17. Kitagawa H, Fujiwara M, Osumi Y. Effects of water-immersion stress on gastric secretion and mucosal blood flow in rats. Gastroenterology. 1979; 77:298–302. PMID: 447044.

18. Konturek PK, Brzozowski T, Konturek SJ, Dembinski A. Role of epidermal growth factor, prostaglandin and sulfhydryls in stress induced gastric lesions. Gastroenterology. 1990; 99(6):1607–1615. PMID: 2227276.
19. Konturek SJ, Brzozowski T, Radecki T. Protective action of omeprazole, a benzimidazole derivative on gastric mucosal damage by aspirin and ethanol in rats. Digestion. 1983; 27:159–164. PMID: 6354810.

20. Kromer W, Postius S, Riedel R, Simon WA, Hanauer G, Brand U, Gönne S, Parsons ME. BY 1023/SK&F96022 Inn pantoprazole, a novel gastric proton pump inhibitor, potently inhibits acid secretion but lacks relevant cytochrome P450 interactions. J Pharmacol Exp Ther. 1990; 254:129–135. PMID: 2164086.
21. Lindberg P, Nordberg P, Alminger T, Brandstrom A, Wallmark B. The mechanism of action of the gastric acid secretion inhibitor omeprazole. J Med Chem. 1986; 29:1327–1329. PMID: 3016260.
22. Lou LX, Geng B, Yu F, Zhang J, Pan CS, Chen L, Qi YF, Ke Y, Wang X, Tang CS. Endoplasmic reticulum stress response in involved in the pathogenesis of stress induced gastric lesions in rats. Life Sci. 2006; 79:1856–1864. PMID: 16875701.
23. Neal MJ. Medical Pharmacology at a Glance. 2003. 3rd ed. London: Blackwell Publishing Inc;p. 30–31.
24. Piotrowski J, Piotrowski E, Skrodzka D, Slomiany A, Slomiany BL. Gastric mucosal apoptosis induced by ethanol: effect ofantiulcer agents. Biochem Mol Biol Int. 1997a; 42(2):247–254. PMID: 9238522.
25. Piotrowski J, Piotrowski E, Skrodzka D, Slomiany A, Slomiany BL. Induction of acute gastritis and epithelial apoptosis by Helicobacter pylori lipopolysaccharide. Scand J Gastroenterol. 1997b; 32(3):203–211. PMID: 9085455.
26. Raffin RP, Colomé LM, Schapoval EE, Jornada DS, Pohlmann AR, Guterres SS. Gastro-resistant microparticles containing sodium pantoprazole: stability and in vivo anti-ulcer activity. Open Drug Deliv J. 2007; 1:28–35.
27. Rao ChV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, Pushpangadan P. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol. 2004; 91:243–249. PMID: 15120446.
28. Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Gastroenterology. 1979; 77:433–443. PMID: 456839.

29. Shah PJ, Gandhi MS, Shah MB, Goswami SS, Santani D. Study of Minusopd elengi bark in experimental gastric ulcers. J Ethnopharmacol. 2003; 89:305–311. PMID: 14611897.
30. Shay M, Komarov SA, Fels D, Meranze D, Gruenstein H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945; 5:43–61.
31. Slomiany BL, Piotrowski J, Slomiany A. Induction of tumor necrosis factor-alpha and apoptosis in gastric mucosal injury by indomethacin: effect of omeprazole and ebrotidine. Scand J Gastroenterol. 1997; 32(7):638–642. PMID: 9246701.
32. Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996; 10:731–740. PMID: 8635690.

33. Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+, K+-ATPase activity. J Biol Chem. 1985; 260(25):13681–13684. PMID: 2997178.
Figure 1
Representative findings of gastric ulcers (arrows) induced by water-immersion restraint stress. Rats were placed in a restraint device, immersed up to their xiphoid process in a 22℃ water bath, and sacrificed 4 hours later for the measurement of ulcer lesions. A, vehicle control; B, 5 mg/kg pantoprazole. Scale bar=1 mm.
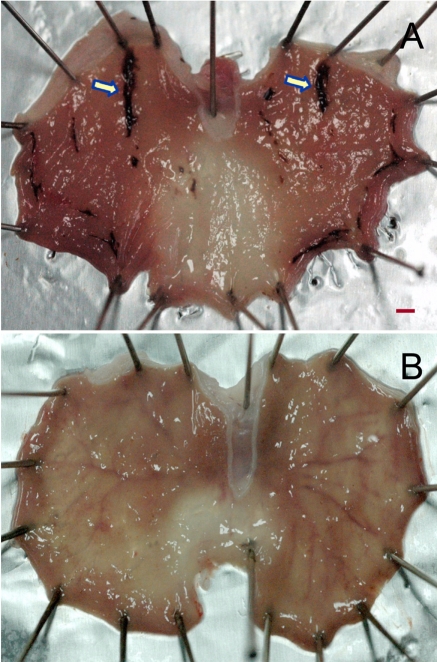
Figure 2
Representative findings of gastric ulcers (arrows) induced by ethanol (1 mL/kg). Rats were orally administered with 1 mL/kg of pure ethanol, and sacrificed 1 hour later for the measurement of ulcer lesions. A, vehicle control; B, 10 mg/kg pantoprazole. Scale bar=1 mm.
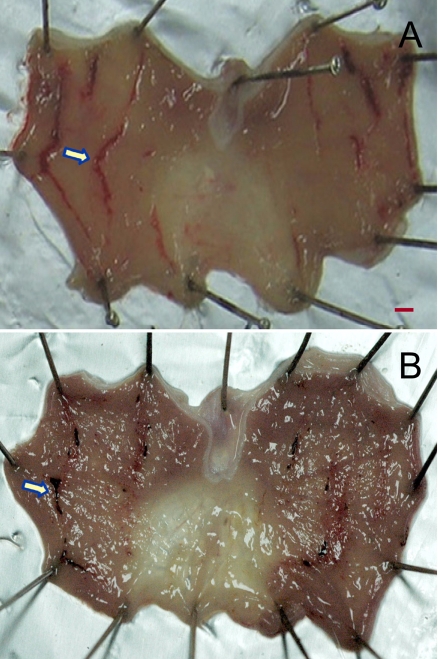
Figure 3
Representative findings of gastric ulcers (arrows) induced by pylorus ligation. Rats were subjected to an operation of pylorus ligation, and sacrificed 17 hours later for scoring of ulcer lesions. A, vehicle control; B, 25 mg/kg pantoprazole.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download