Abstract
This study was developed and validated for the determination of oxyclozanide residue concentrations in beef and commercial milk, using high-performance liquid chromatography system. Oxyclozanide was successfully separated on a reverse phase column (Xbridge-C18, 4.6×250 mm, 5 µm) with a mobile phase composed of acetonitrile and 0.1% phosphoric acid (60:40, v/v%). This analytical procedure involved a deproteinization process using acetonitrile for beef and 2% formic acid in acetonitrile for commercial milk, dehydration by adding sodium sulfate to the liquid analytical sample, and a defatting process using n-hexane; after these steps, the extract was exposed to a stream of nitrogen dryness. The final extracted sample was dissolved in the mobile phase and filtered using a 0.45 µm syringe filter. This method had good selectivity and recovery (70.70±7.90-110.79±14.95%) from the matrices. The LOQs ranged from 9.7 to 9.8 µg/kg for beef and commercial milk. The recoveries met the standards set by the CODEX guideline.
Oxyclozanide [2,3,5-trichloro-N-(3,5-dichloro-2-hydroxyphenyl)-6-hydroxybenzamide] is a salicylanilide anthelmintic that mainly acts by uncoupling oxidative phosphorylation in flukes (Froyd, 1968; Corbett and Goose, 1971; Veenendaal and de Waal, 1974). It is used in the treatment and control of fascioliasis in ruminants, especially domestic animals such as cattle, sheep, and goats (Walley, 1966; Walley, 1970; Paraud et al, 2009). Several earlier reports have suggested that oxyclozanide residues present in animal food products such as beef and milk (Caldow et al, 2009; Sakamoto et al, 2020; Cooper et al, 2011). EU (European Medicines Agency; http://www.emea.europa.eu) and Japan have already established maximum residue limits (MRLs) for oxyclozanide in animal tissues and products, including muscle, fat, kidney, liver, and milk. However, little effort has been made to develop an effective method to determine the concentrations of oxyclozanide residues in biomatrices (Takeba et al, 1996; Khan et al, 2000). In the Republic of Korea, oxyclozanide was approved for use in veterinary medicine many years ago; however, there have been no MRL regulations set. Moreover, no analytical method has been developed to determine the residual quantities of the drug in various matrices such as beef. Therefore, this study was performed to develop an analytical method to determine the residual concentration of oxyclozanide in beef muscle and commercial whole milk. The method described herein uses high-performance liquid chromatography coupled with UV absorption spectrometry (HPLC-UVD).
Standard oxyclozanide was supplied from Dr. Ehrenstofer (GmbH, Auhsburg, Germany). Acetonitrile, n-hexane, and methanol HPLC-grade solvents were provided by J.T. Baker (Griesheim, Germany). Formic acid was obtained from Fluka (Buchs, Switzerland) and sodium sulfate from Junsei (Tokyo, Japan). All other chemicals and solvents were of analytical grade, unless stated otherwise. Distilled water (DW) prepared for HPLC was passed through 18 MΩ/cm Clxxxdim1 (ELGA, High Wycombe, UK) before use. Beef and commercial whole milk were purchased from commercial markets.
Stock solution of 100 µg/mL oxyclozanide was prepared in acetonitrile. The stock solution was stored at 4℃. The working solutions for HPLC analysis were prepared daily from the stock solution.
The blank or spiked beef samples were chopped up, and commercial milk samples were homogenized. Ten grams (approximately 10 mL of milk) of each blended sample was transferred to a separate 50 mL polypropylene centrifuge tube. After a 10 min equilibration period, 20 mL of acetonitrile was added, and the sample was vortex-mixed (10 sec) and left undisturbed for 10 min. For milk samples, 10 g of anhydrous sodium sulfate was added thereafter, and the sample was thoroughly mixed for 10 min. The samples were centrifuged at 2,700 g for 20 min. The samples were then extracted once again with 20 mL of acetonitrile. The organic layers were recovered after 20 min of centrifugation at 2,700 g. n-Hexane (20 mL) was then added to the combined supernatant. After shaking 5 times, the upper layer was discarded after 20 min of centrifugation at 2,700 g. The lower layers were transferred to a 15 mL polypropylene centrifuge tube and concentrated under a stream of nitrogen at 50℃ (0.5-1mL). The concentrated residues were then dissolved in 2 mL of acetonitrile, vortex mixed (15 sec), and centrifuged at 16,000 g for 20 min. The upper layers were placed in a new tube and concentrated under a stream of nitrogen at 50℃ (0.5-1mL). The concentrated residues were then dissolved in 200 µL of acetonitrile. The upper layers were filtered through a 0.45 µm GHP syringe filter (Whatman) after 20 min of centrifugation at 16,000 g (4℃).
The levels of oxyclozanide were determined using an HPLC system (Agilent series 1100, Palo Alto, CA, USA) equipped with a quart pump (G1311A), an autosampler (G1313A), a degasser (G1322A), a column oven (G1316A), and a UV detector (1321A) operated at 300 nm. The samples were separated on an ODS column (Waters Xbridge C18, 4.6×250 mm, 5 µm) and were eluted with a mobile phase consisting of a mixture of acetonitrile and 0.1% phosphoric acid (4:6, v:v). The isocratic mode was run at a flow rate of 1 mL/min, and aliquots of 50 µL were injected onto the column. The column temperature was maintained at 40℃.
The method was validated to fulfill the criteria specified by the CODEX guidelines for specificity, linearity, LOD and LOQ, precision, and recovery (or accuracy) [CODEX Alimentarius Commission (1993); CODEX Guidelines for the Establishment of a Regulatory Programme for Control of Veterinary Drug Residues in Foods, Part III. Attributes of analytical Methods for Residue of Veterinary Drugs in Foods; http://www.codexalimentarius.net]. Blank samples (beef and commercial milk) were evaluated for matrix interference. Linearity was evaluated for each sample (beef and commercial milk) spiking the samples with six levels of oxychlozanide [0.5 (0.75 in milk), 1, 2, 3, 4, and 5 times the permitted limit (MRL)]. Each sample was analyzed 4 times. Calibration curves were calculated via least-squares linear regression analysis of the peak area ratio of each analyte to the pure standard solution. The calculations for the LOD were based on the SD of the y-intercepts of the regression (σ) analysis and the slope (S) using the equation LOD=3.3 σ/S. The LOQ was calculated via the equation LOQ=10 σ/S. Recovery was assessed using samples 0.5, 1, and 2 times the permitted limit. Six samples were prepared for each concentration. The responses of the oxyclozanide added to blank samples before extraction were compared with those to which oxyclozanide was added after extraction (control).
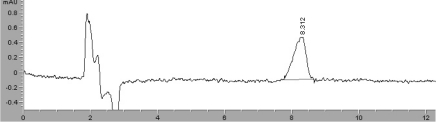
To optimize the separation of the analytes, the components and the relative percentages of these components in the mobile phase were tested. A linear gradient mobile phase consisting of acetonitrile/0.1% phosphoric acid was used. Oxyclozanide was eluted at 8.3 min under isocratic conditions of acetonitrile/0.1% phosphoric acid (60:40, v/v). The excitation/emission wavelengths used for the UV detection were 300 nm for oxyclozanide. Column temperatures of 35 and 40℃ were tested. The oxyclozanide peak was resolved at 40℃. The adjacent peak pH is one of the most powerful tools for optimizing the separation of analyte mixtures. To optimize the pH of the mobile phase, three different pHs (2.5, 3.5, and 4.5) were tested. Good separation was obtained at pH 3.5. A typical chromatogram corresponding to an oxyclozanide standard is shown in Figure 1. The separation of the analytes was achieved in less than 10 min. The retention time was 8.3 min for oxyclozanide.
The majority of the methods reported for the analysis of oxyclozanide residues in tissue involve protein precipitation with an organic solvent in the presence of either an acid or a base. The most commonly employed organic solvents are acetonitrile, ethanol, and methanol, with acetonitrile being the most frequently used. In this study, the analytes were extracted from a complex matrix after deproteinization with acetonitrile. Acetonitrile was effective in the deproteinization of beef and commercial milk, and in the isolation of analytes from spiked samples.
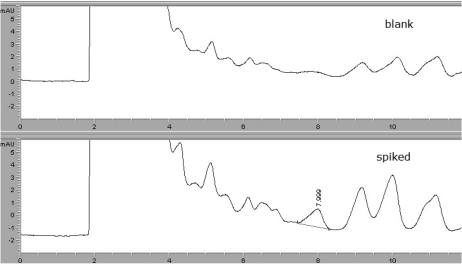
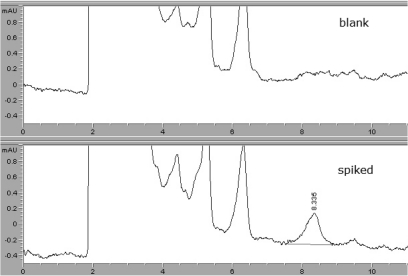
The specificity of the method utilized for each sample was evaluated by analyzing blank samples. None of the blanks evidenced any interference from beef or milk. Oxyclozanide was extracted successfully from beef and milk samples (Figures 2 and 3).
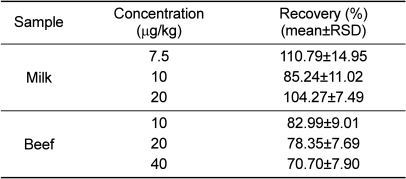
The chromatographic method was shown to have a linear response over six concentration levels [0.5 (0.75 in milk), 1, 2, 3, 4, and 5 times the permitted limit]. Each oxyclozanide level was repeated 4 times (r>0.984), and each sample was analyzed five times. Calibration curves were calculated via least-squares linear regression analysis of the peak area ratio of each analyte to the pure standard solution. The LOQs ranged from 9.7 to 9.8 µg/kg in beef and commercial milk. In all cases, the LOQs were lower than the maximum residue limits suggested by the Korea Food and Drug Administration for these compounds in the tested matrices (Table 1).
The accuracy and precision of the method developed herein were expressed in terms of recovery. The recoveries (mean±RSD%) are summarized in Table 2. All matrices were analyzed at concentrations of 0.5 (0.75 in milk), 1, and 2 times the limits permitted by the CODEX guideline. The mean recovery rates of oxyclozanide in the spiked samples were 70.70±7.90 to 110.79±14.95%.
In this study, an analytical protocol involving a one-step extraction followed by LC was successfully developed. In terms of accuracy, no extract cleanup was deemed necessary. The developed method has acceptable accuracy (recovery) and precision. This procedure is simple and allows for the determination of the concentrations of residues of this compound in different matrices with a high degree of sensitivity.
Acknowledgments
This study was supported by the Korea Food and Drug Administration (09062KFDA011) in 2009.
References
1. Caldow M, Sharman M, Kelly M, Day J, Hird S, Tarbin JA. Multiresidue determination of phenolic and salicylanilide anthelmintics and related compounds in bovine kidney by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2009; 1216:8200–8205. PMID: 19426989.

2. Cooper KM, Whelan M, Danaher M, Kennedy DG. Stability during cooking of anthelmintic veterinary drug residues in beef. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011; 28(2):155–165. PMID: 21240825.

3. Corbett JR, Goose AJ. The biochemical mode of action of the fasciolicides nitroxynil, hexachlorophane and oxyclozanide. Biochem J. 1971; 121(3):41P. PMID: 5165621.

4. Froyd G. Field trials with oxyclozanide. A new liverfluke remedy for sheep and cattle. Br Vet J. 1968; 124:116–125. PMID: 5689150.
5. Khan AR, Akhtar MJ, Mahmood R, Ahmed SM, Malook S, Iqbal M. LC assay method for oxfendazole and oxyclozanide in pharmaceutical preparation. J Pharm Biomed Anal. 2000; 22:111–114. PMID: 10727129.
6. Paraud C, Gaudin C, Pors I, Chartier C. Efficacy of oxyclozanide against the rumen fluke Calicophoron daubneyi in experimentally infected goats. Vet J. 2009; 180:265–267. PMID: 18314360.

7. Sakamoto M, Takeba K, Sasamoto T, Kusano T, Hayashi H, Kanai S, Kanda M, Nagayama T. Determination of bithionol, bromophen, nitroxynil, oxyclozanide, and tribromsalan in milk with liquid chromatography coupled with tandem mass spectrometry. J AOAC Int. 2010; 93:1340–1346. PMID: 20922970.

8. Takeba K, Itoh T, Matsumoto M, Nakazawa H, Tanabe S. Simultaneous determination of five fasciolicides in milk by liquid chromatography with electrochemical detection. J AOAC Int. 1996; 79:848–852. PMID: 8757441.

9. Veenendaal G H, de Waal MJ. Uncoupling activity of the anthelmintic oxyclozanide in rodents. Br J Pharmacol. 1974; 50:435–437. PMID: 4277750.
10. Walley JK. Oxyclozanide (3,3',5,5',6-pentachloro-2,2'-dihydroxybenzanilide--6Zanil") in the treatment of the liver fluke Fasciola hepatica in sheep and cattle. Vet Rec. 1966; 78:267–276. PMID: 5948202.
11. Walley JK. Tetramisole and oxyclozanide in combination in the treatment of gastro-intestinal worms, lungworms and liver fluke (Fasciola hepatica) in sheep, goats and cattle. Vet Rec. 1970; 86:222–227. PMID: 5434426.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download