Abstract
Sarcocystis spp is a causative agent of sarcocystosis. They have a characteristic life cycle infecting both prey and predator. Sarcocystis can cause myositis, atrophy of the adjacent cells and abortion in cattle. In mice, sarcocystosis causes mild cellular reactions without clinical disease. Severe haemorrhage and abortion were also reported. For monitoring the disease in wild rodents of the Korean peninsula, we captured Apodemus agrarius chejuensis on Jeju island and examined the specimen histopathologically. Intramuscular cysts were found and diagnosed as Sarcocystis. Sarcocystic infection has been reported in worldwide. There have been many reported infections in cattle and pigs in Korea. To our knowledge, this is the first report of Sarcocystis in Apodemus agrarius chejuensis captured in Korea.
Sarcocystosis is a parasitic disease caused by Sarcocystis spp [1]. In 1843, cystic intramuscular inclusions were first reported by Miescher, who found 'Miescher's tubes' in the skeletal muscles of the house mouse Mus musculus caught in his home in Switzerland [2,3]. Miescher's tubules are late generation meronts or sarcocysts, and, when mature, are filled with bradyzoites [2].
Genus Sarcocystis belongs to the Family Sarcocystidae. They have a two-host life cycle involving mainly herbivores and omnivores as intermediate hosts and carnivores as the definitive host [4]. When a predator eats an animal, the bradyzoites become gamonts in the cells of their intestinal wall, which form gametes, and fertilization takes place resulting in zygotes from oocysts. The oocysts sporulate in the host's intestine and sporulated sporocysts are passed in the feces. Then, the sporocysts are eaten by the prey animals and the sporozoites enter the bloodstream and become first generation meronts in the endothelial or subendothelial cells of the blood vessels. The meronts produce first generation merozoites, which enter new endothelial or subendothelial cells and become second generation meronts. The latter, second generation merozoites enter the muscle and become third generation meronts [2]. Sarcocystis can cause myositis, pressure atrophy of the adjacent cells, abortion, clinical illness and even death [3,5]. Human beings act as the definitive host for two zoonotic species, namely Sarcocystis hominis and Sarcocystis suihoimini and can be the intermediate host for Sarcocystis lindemanni [4,6].
For diagnosis of sarcocystis, microscopic examination using the muscle squash method, peptic digestion and histological tests are used for surveillance in slaughtered cattle or farmed elk [7,8]. Serological tests using enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent antibodies (IFAT) have also been used for humans [9]. For the investigation in wild rodents and laboratory mice, identification of cysts with macroscopic and microscopic examination in tissue has usually been used [3,10].
Sarcocystis have been found in various animals including moose, roe deer (Capreolus capreolus), coyotes (Canis latrans), mule deer (Odocoileus hemionus hemionus), sheep, horse and Corvid birds [11-14]; it is distributed worldwide including Korea, Norway, Canada, Iraq and the United states [8,10,13-16].
In the past, sarcocystis was found in 4% of wild mice in a laboratory area in 1978 and commonly observed in laboratory mice [17,18]. Cat was known to have shed sporocysts of Sarcocystis muris and coprophagous insects (cockroaches) as transport hosts [3].
In August 2010, we performed a surveillance project to monitor the disease of wild rodents in the Korean peninsula. Brain, heart, lung, liver, spleen, stomach, intestine and kidney were collected and fixed in 10% neutral buffered formalin for 48 h. After embedding in paraffin, sections were prepared at 4 ìm thickness were stained with haematoxylin and eosin, and observed microscopically.
We found cyst in muscle tissue of a female Apodemus agrarius chenuensis captured in Jeju island (N33°20'33.8", E126°20'12.1", body weight 37.3 g, body length 107.84 mm) after histopathological examination. At necropsy, a gross lesion was not detected.
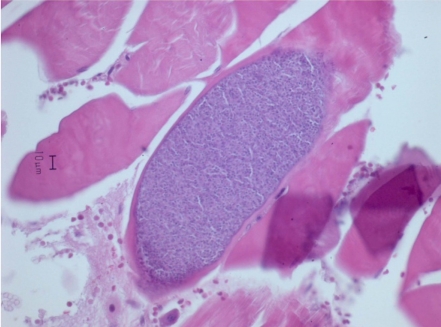
Cysts were oval or elongated, being 64-98 µm wide (mean 84 µm) and 201-346 µm long (mean 255 µm: Figure 1). Cysts were well demarcated with adjacent muscular tissue. There were no inflammatory reactions or migration of leukocytes in the surrounding tissue. We diagnosed this as Sarcocystis based on the shape and size of cysts.
Many kinds of Sarcocystis including Sarcocystis proechimyos, Sarcocystis oryzomyos, Sarcocystis azevedoi, Sarcocystis marmosae, Sarcocystis garnhami and Sarcocystis muris infect rodents [8]. In the mouse, Sarcocystis has been found in the skeletal muscle and less commonly in the cardiac, oesophageal and diaphragmatic muscle and it usually causes only a mild cellular reaction and no clinical disease. Histologically, infected Sarcocystis muris cysts were either elongated or circular with a mean measuring size of 254×24.5 µm. The wall is 2.5 µm in thickness [3]. There was no leukocytic infiltration present in the adjacent muscle tissue [1,8]. For differential diagnosis, other intramuscular parasites can be easily distinguished by size of the cyst, structures and induced inflammatory reactions histologically [3]. To our knowledge, this is the first report of Sarcocystis in Apodemus agrarius chejuensis captured in Korea.
Acknowledgments
All authors appreciate support from the National Institute of Environmental Research. This study was partially supported by the Research Institute for Verterinary Science, Seoul National University.
References
1. Latif B, Vellayan S, Omar E, Abdullah S, Mat Desa N. Sarcocystosis among wild captive and zoo animals in Malaysia. Korean J Parasitol. 2010; 48(3):213–217. PMID: 20877499.

2. Levine ND. The taxonomy of Sarcocystis (protozoa, apicomplexa) species. J Parasitol. 1986; 72(3):372–382. PMID: 3091802.
3. Tillmann T, Kamino K, Mohr U. Sarcocystis muris-a rare case in laboratory mice. Lab Anim. 1999; 33(4):390–392. PMID: 10778789.
4. Noh JW, Jang DH, Kang YB, Jang H, Wee SH. Effects of temperature on viability of sarcocysts of Sarcocystis cruzi in cardiac muscle of cattle. Korean J Vet Public Health. 1988; 12(2):151–155.
5. Wee SH, Shin SS. Experimental induction of the two-host life cycle of Sarcocystis cruzi between dogs and Korean native calves. Korean J Parasitol. 2001; 39(3):227–232. PMID: 11590912.
7. Gjerde B. Ultrastructure of the cysts of Sarcocystis grueneri from cardiac muscle of reindeer (Rangifer tarandus tarandus). Z Parasitenkd. 1985; 71(2):189–198. PMID: 3922150.
8. Kang SS, Yi YJ, Cui XS, Kwon YB, Cho SK, Choi SH. Sarcocystis infection in farmed elk (Cervus canadensis). Korean J Vet Clin Med. 1999; 16(2):529–532.
9. Habeeb YS, Selim MA, Ali MS, Mahmoud LA, Abdel Hadi AM, Shafei A. Serological diagnosis of extraintestinal sarcocystosis. J Egypt Soc Parasitol. 1996; 26:393–400. PMID: 8754648.
10. Shaw JJ, Lainson R. Sarcocystis of rodents and marsupials in Brazil. Parasitology. 1969; 59(1):233–244. PMID: 4976872.

11. Davis CR, Barr BC, Pascoe JR, Olander HJ, Dubey JP. Hepatic sarcocystosis in a horse. J Parasitol. 1999; 85(5):965–968. PMID: 10577737.

12. Dubey JP, Lindsay DS, Speer CA, Fayer R, Livingston CW Jr. Sarcocystis arieticanis and other Sarcocystis species in sheep in the United States. J Parasitol. 1988; 74(6):1033–1038. PMID: 3142990.
13. Colwell DD, Mahrt JL. Ultrastructure of the cyst wall and merozoites of Sarcocystis from moose (Alces alces) in Alberta, Canada. Z Parasitenkd. 1981; 65(3):317–329. PMID: 6797140.
14. Gjerde B, Dahlgren SS. Corvid birds (Corvidae) act as definitive hosts for Sarcocystis ovalis in moose (Alces alces). Parasitol Res. 2010; 107(6):1445–1453. PMID: 20697910.
15. Atkinson CT, Wright SD, Telford SR Jr, McLaughlin GS, Forrester DJ, Roelke ME, McCown JW. Morphology, prevalence, and distribution of Sarcocystis spp. in white-tailed deer (Odocoileus virginianus) from Florida. J Wildl Dis. 1993; 29(1):73–84. PMID: 8445792.
16. Moon MH. Sarcocystis infection and identification of Sarcocystis species in pigs in Korea. Korean J Vet Res. 1989; 29(3):325–331.
17. Smith DD, Frenkel JK. Cockroaches as vectors of Sarcocystis muris and of other coccidia in the laboratory. J Parasitol. 1978; 64(2):315–319. PMID: 417163.
18. Ruiz A, Frenkel JK. Recognition of cyclic transmission of Sarcocystis muris by cats. J Infect Dis. 1976; 133(4):409–418. PMID: 816973.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download