Abstract
An eleven-year-old spayed female Yorkshire Terrier presented with a sublumbar mass and upon ultrasonographic examination, was revealed to have a mammary gland tumor. Black to reddish colored masses, located in the visceral peritoneum of the sublumbar region was observed on laparotomy with masectomy of the right side. In the laparotomy, we observed reddish masses multifocally located in the serosal membrane of the large intestine. Histopathologic examination of the intestinal and abdominal mass showed highly invasiveness into the muscle and metastasis of melanocytic tumor cells through the blood vessels. The mammary glands showed abnormal hyperplasia of melanocytes, destruction of the normal glands by tumor cells and infiltration of some lymphocytes in the pool of melanocytic cells. We have identified a malignant melanoma containing an angiotumoral complex in which tumor cells occupied a pericytic location along the microvessels with intravasation determined by immunohistochemistry for S100 protein and protein kinase C-α. Histologic findings in this dog lead to a diagnosis of an angiotropic metastatic malignant melanoma.
Melanoma is relatively common in dogs, accounting for 3% of all neoplasms and up to 7% of all malignant tumors [1]. Canine malignant melanoma (CMM) is a spontaneous, aggressive, rare and metastatic neoplasm. CMM of the oral cavity, nail bed, foot pad and mucocutaneous junction is a spontaneously occurring, highly aggressive and frequently metastatic neoplasm [1-4]. Canine oral melanomas are virtually always considered malignant tumors, whereas more than 95% of cutaneous melanocytic lesions are benign [5]. Dermal melanomas in dogs generally follow a benign course. Canine patients with advanced diseases (WHO stage II, III, or IV) have reported median survival times of <5 months with aggressive local excision [1-3]. Unfortunately, response rates to chemotherapy in dogs with advanced melanoma range from 8% to 28% with little evidence that treatment improves survival [6-8]. Therefore, understanding the factors that contribute to tumor growth and metastatic dissemination is of paramount importance for the design and effective use of novel therapeutic strategies to combat tumor growth and spread. The propensity for malignant melanoma to migrate along anatomical structures such as nerves (neurotropism) and skin appendages has been recognized as a common phenomenon for many years [9]. The mechanism of melanoma metastasis in animals is as yet unclear, although previous studies have reported mechanisms of extravascular migratory metastasis and anti-tumoral complex [9-13]. To our knowledge, CMM with metastasis into the internal organs are rare, but we present an angiotropic metastatic malignant melanoma of a dog with detailed histopathological findings using immunohistochemistry.
The masectomized tissue of an 11-year-old female Yorkshire Terrier with large intestinal and abdominal tissues were obtained from a Hwanggum Animal Medical Center (Daegu, Korea) for evaluation of tumors. Radiographs revealed abdominal masses in the sublumbar region. A laparatomy revealed masses that were black to reddish colored and 2-3 mm in diameter; they were multifocally located on the serosal membrane of the large intestine and visceral peritoneum of the sublumbar region. The subcutaneous lesion of the right mammary gland showed a black to reddish mass with black to reddish petechia and ecchymosis.
Tissues samples for light microscopy were fixed in 10% neutral buffered formalin, paraffin embedded, and stained with hematoxylin and eosin (H&E). For immunohistochemistry, tissue sections were deparaffinized in xylene, rehydrated in graded alcohol series, incubated in a solution of 0.3% hydrogen peroxide in methanol for 30 minutes and microwaved at 750W for 10 min in 10 mmol/L citrate buffer, pH6.0. Tissue sections were washed with phosphate-buffered saline (PBS) and then immunostained with primary antibody. The primary antibodies used recognized S100 protein (diluted in 1:200, DakoCytomation, Carpinteria, CA, USA), vimentin (diluted in 1:100, DakoCytomation), protein kinase C-α (PKC-α; diluted in 1:100, Santa Cruz Biotechnogy, Santa Cruz, CA, USA). The avidin-biotin-peroxidase complex (ABC) solution of the ABC kit (Vector Laboratories, Burlingame, CA, USA) with 3,3-diaminobenzidine (Zymed Laboratories, San Francisco, CA, USA) was used for detection. Tissue sections were then rinsed in distilled water and counterstained with Mayer's hematoxylin.
The most extensively invaded lesions, skin and mammary glands showed abnormal hyperplasia of melanocytes in the dermal layers with hyperactivated epidermis melanocytes (Figure 1A). Melanocytic tumor cells had invaded into the dermal lymphatic channels (Figure 1B) and micro vessels and were hyperpigmented in the dermal reticular layer and deeper layers. Mammary glands were also heavily pigmented with mixed round and epithelioid cells. Normal mammary glands were invaded and destroyed by tumor cells (Figures 1C and 1D). Pleomorphic round cells were arranged in sheets or clusters. There were also myoepithelial cells exhibiting hyperplasia. Some lymphocytes had infiltrated the pool of melanocytic cells. Histologic evaluation of the mass in the sublumbar region revealed melanocytes intermingled with abdominal connective tissue and invasiveness of the micro-vessels (Figures 1E and 1F). The neoplastic cells were fusiform and epithelioid in the peritoneum. In a section of the large intestinal mass, melanocytes had invaded the muscular layer and the metastasis of melanocytic tumor cells through the blood vessels (Figures 1G and 1H).
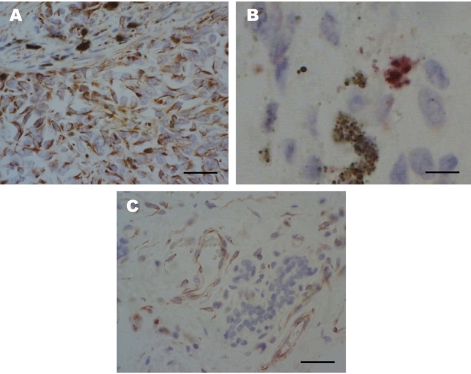
Immunohistochemically, mononuclear amelanotic cells were positive for cytoplasmic vimentin staining (Figure 2A) and multinucleated cells containing melanin granules were positive for intranuclear and cytoplasmic S100 staining (Figure 2B). The tumor cells are closely associated with the external surfaces of microvessels which expressed PKC-α (Figure 2C) and weakly positive for PKC-ε. Our interpretation of these observations was that the multifocally invaded tumor, classified as a malignant melanoma, had at least three distinct lesions with very similar hyperplasia types and metastasis into blood vessels.
Melanoma may be one of the few neoplasms in animals whose location is an important prognostic indicator in its own right [5]. There is no obvious relationship between histologic characteristics, including mitotic figures and pigmentation, and survival rate [4,14]. The initial definitive diagnosis of melanoma is usually done by histologic evaluation, with cytopathology used as a screen before biopsy or as an adjunct to biopsy [15]. Morphology of the melanoma in the metastatic site is frequently similar to the primary tumor. Distant sites are sometimes less pigmented and amelanotic epithelioid in form. Vascular and/or lymphatic invasion has been thought to be a direct manifestation of metastasis for progress and prognostic factors. Angiotropism in malignant melanoma has been observed in previous studies by conventional microscopy in routine tissue sections in human [13,16-19]. To our knowledge, there have been no reports of the origin of the potential mechanism of melanoma. We evaluated the expression of vimentin, S100 protein and PKC-α. S100 staining characteristics have also been useful in the diagnosis of canine amelanotic melanomas [19,20]. PKC was identified as a signaling protein involved in the carcinogenesis of skin tumors [21]. The α-isozyme of PKC is widely expressed in tissues and abnormal levels are found in tissues such as proliferating pulmonary arteriolar smooth muscle cells and adventitial fibroblasts after chronic exposure to hypoxia in mammals [22-26]. In our study, we carefully had to distinguish angiotropism from entrapment or engulfment of vessels by the melanoma. PKC-α activation appeared in the epithelium of microvascular channels and some tumor cells at the periphery of the vessels. There are a number of known activation signals for PKC-α such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and fibroblast growth factor (FGF) [27]. Although the latter feature may in fact constitute angiotropism and metastasis, we have no means at present to verify if and when such entrapment is biologically significant. From our results, there was also vascular/lymphatic invasion, which has been thought to be a direct manifestation of metastasis progress. Therefore, we consider the significance of recording vascular/lymphatic invasion until further studies are better able to sort out the problems of angiotropism versus a true intraluminal tumor.
Although our study involved only a single case of melanoma, it is of interest that the angiotropic melanoma presented had detailed, interesting findings of histopathology and immunohistochemistry. These results showed that black masses of the serosal membrane of the large intestine and peritoneum originated from the skin. The metastasis of most tumors usually occurs in the lung and other solid organs in clinical cases. In particular, the serosal membrane and peritoneum are susceptible to negligible lesion. This report describes the occurrence of an angiotropic metastatic melanoma in the serosal membranes of the large intestine rather than the internal regions of the solid organ.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0029642) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0025973) and Hallym Academy of Sciences at Hallym University in Korea (HRF-2009-039).
References
1. Bostock DE. Prognosis after surgical excision of canine melanomas. Vet Pathol. 1979; 16(1):32–40. PMID: 462717.

2. Harvey HJ, MacEwen G, Braun D, Patnaik AK, Withrow SJ, Jongeward S. Prognostic criteria for dogs with oral melanoma. J Am Vet Med Assoc. 1981; 178(6):580–582. PMID: 7263464.
3. MacEwen EG, Patnaik AK, Harvey HJ, Hayers AA, Matus R. Canine oral melanoma: comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Invest. 1986; 4(5):397–402. PMID: 3801954.

4. Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, Kottler SJ. Reprospective study of 338 canine oral melanomas with clinical, histologic, and immunohistichemical review of 129 cases. Vet Pathol. 2000; 37(6):597–608. PMID: 11105949.
5. Smith SH, Goldschmidt MH, McManus PM. A comparative review of melanocytic neoplasms. Vet Pathol. 2002; 39(6):651–678. PMID: 12450197.

6. Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, Beqq CB, Aqarwala SS, Schuchter LM, Ernstoff MS, Houghton AN, Kirkwood JM. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999; 17(9):2745–2751. PMID: 10561349.

7. Houghton AN, Meyers ML, Chapman PB. Medical treatment of metastatic melanoma. Surg Clin North Am. 1996; 76(6):1343–1354. PMID: 8977555.

8. Rassnick KM, Ruslander DM, Cotter SM, Al-sarraf R, Bruyette DS, Gamblin RM, Meleo KA, Moore AS. Use of carboplatin for treatment of dogs with malignant melanoma: 27 casses (1989-2000). J Am Vet Med Assoc. 2001; 218(9):1444–1448. PMID: 11345308.
9. Barnhill RL. The biology of melanoma micrometastases. Recent Results Cancer Res. 2001; 158:3–13. PMID: 11092028.
10. Lugassy C, Barnhill RL, Christensen L. Melanoma and extravascular migratory metastasis. J Cutan Pathol. 2000; 27(9):481. PMID: 11028822.
11. Lugassy C, Eyden BP, Christensen L, Escande JP. Angiotumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol. 1997; 29(1):19–28. PMID: 9066138.
12. Lugassy C, Dickersin GR, Christensen L, Karaoli T, LeCharpentier M, Escande JP, Barnhill RL. Ultrastructural and immunohistochemical studies of the periendothelial matrix in human melanoma: evidence for an amorphous matrix containing laminin. J Cutan Pathol. 1999; 26(2):78–83. PMID: 10082397.

13. Moreno A, Espanol I, Ramogosa V. Angiotropic malignant melanoma. Report of two cases. J Cutan Pathol. 1992; 19(4):325–329. PMID: 1430472.

14. Eisen D, Voorhees JJ. Oral melanoma and other pigmented lesions of the oral cavity. J Am Acad Dermatol. 1991; 24(4):527–537. PMID: 2033125.

15. Griffiths GL, Lumsden JH. Fine neddle aspiration cytology and histologic correlation in canine tumors. Vet Clin Pathol. 1984; 13(1):13–17. PMID: 15311390.
16. Kerr S, Going JJ. Angiocentric invasion by lentigo malignat melanoma. J Clin Pathol. 1994; 47(2):183–184. PMID: 8132839.
17. Shea CR, Kline MA, Lugo J, McNutt NS. Angiotropic metastatic malignant melanoma. Am J Dermatopathol. 1995; 17(1):58–62. PMID: 7695012.

18. Saluja A, Money N, Zivoney DI, Solomon AR. Angiotropic malignant melanoma: A rare pattern of local metastases. J Am Acad Dermatol. 2001; 44(5):829–832. PMID: 11312432.

19. Rabanal RH, Fondevila DM, Montane V, Domingo M, Ferrer L. Immunocytochemical diagnosis of skin tumours of the dog with special reference to undifferentiated types. Res Vet Sci. 1989; 47(1):129–133. PMID: 2475897.

20. Sandusky GE Jr, Carlton WW, Wightman KA. Immunohistochemial staining for S100 protein in the diagnosis of canine amelanotic melanoma. Vet Pathol. 1985; 22(6):577–581. PMID: 2417399.
21. Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988; 334(6184):661–665. PMID: 3045562.

22. Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol Ther. 1993; 59(3):257–280. PMID: 8309991.

23. Denham DW, Franz MG, Denham W, Zervos EE, Gower WR Jr, Rosemurgy AS, Norman J. Directed antisense therapy confirms the role of protein kinase C-α in the tumorigenicity of pancreatic cancer. Surgery. 1998; 124(2):218–223. PMID: 9706141.

24. Gescher A. Towards selective pharmacological modulation of protein kinase C-opportunities for the development of novel anti-neoplastic agents. Br J Cancer. 1992; 66(1):10–19. PMID: 1637658.
25. Cornford P, Evans J, Dodson A, Parsons K, Woolfenden A, Neoptolemos J, Foster CS. Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am J Pathol. 1999; 154(1):137–144. PMID: 9916928.

26. Tan X, Liu YJ, Li JC, Pan JQ, Sun WD, Wang XL. Activation of PKC and pulmonary vascular remodelling in broilers. Res Vet Sci. 2005; 79(2):131–137. PMID: 15924930.

27. Lahn MM, Sundell KL. The role of protein kinase C-α (PKC-α) in melanoma. Melanoma Res. 2004; 14(2):85–89. PMID: 15057036.
Figure 1
Histopathological findings of the most extensively invaded lesions, skin and mammary glands. (A) Primary invasive melanoma. Abnormal hyperplasia of melanocytes in the dermal layers with hyperactivated epidermis melanocytes. H&E stain. Scale bar=500 µm. (B) Primary invasive melanoma. Note melanoma cells closely opposed to the external surface of microvessel (inset); melanocytic tumor cells invaded into the dermal lymphatic follicle and are hyperpigmented in the dermal reticular layer and more deep layers. H&E Stain. Scale bar=200 µm, (inset, H&E stain; Scale bar=500 µm). (C) Mammary glands also heavily pigmented. Abnormal hyperplasia of melanocytes as well as invasiveness and destruction of normal mammary glands by spindle melanocytic tumor cells. H&E stain. Scale bar=500 µm. (D) Mammary glands also heavily pigmented. Normal mammary glands were invaded with destruction by tumor cells. Pleomorphic round cells arranged in sheets or clusters. H&E stain. Scale bar=200 µm. (E) Malignant melanoma of the peritoneum lesion. Invasiveness and metastasis of melanocytic tumor in abdominal connective tissue and blood vessels. H&E stain. Scale bar=500 µm. (F) Malignant melanoma of the peritoneum lesion. Epitheloid and fusiform neoplastic cells infiltration. H&E stain. Scale bar=200 µm. (G and H) Malignant melanoma of the large intestinal lesion. Invasiveness into the muscle and metastasis of melanocytic tumor cells through the blood vessels and lymphatic channels surrounding glandular ducts. H&E stain. Scale bar=500 µm (G) and 200 µm (H).
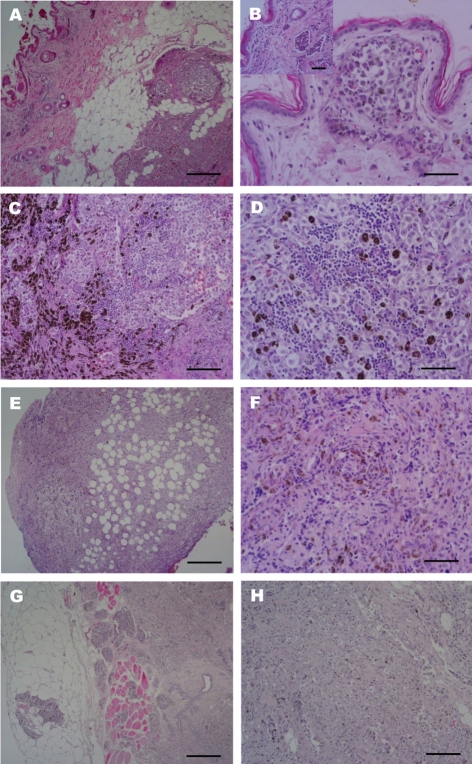
Figure 2
Immunohistochemical stain with antibody for vimentin, S100 and PKC-α. (A) Malignant melanoma of the skin. Vimentin expression of mononuclear epitheloid amelanotic melanoma cells. ABC method, Mayer's hematoxylin counterstain. Scale bar=200 µm. (B) Malignant melanoma of the skin. S100 protein immunoreactivity is detected in melanoma cells, which is surrounded by multiple S100-negative epitheloid-shaped cells. ABC method, Mayer's hematoxylin counterstain. Scale bar=50 µm. (C) Malignant melanoma of the skin. Immunodetection of PKC-α in metastatic malignant melanoma. PKC-α expression in the epithelium of microvessels and some peripheral tumor cells. ABC method, Mayer's hematoxylin counterstain. Scale bar=200 µm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download