Abstract
Suaeda asparagoides (Miq.) has long been used as a Korean folk herbal medicine for the treatment of functional gastrointestinal disorders. However, reports on its pharmacological activity on gastrointestinal motility are scarce. The present study investigated the effects of Suaeda asparagoides water fraction of the extract (SAWF) on antral motility in vitro. Muscle strips from rat gastric antrum were set up in an organ bath in a circular orientation. SAWF (100 µg/mL) inhibited the spontaneous contraction of antral circular muscle strips. These inhibitory effects were not significantly affected by tetrodotoxin (1 µM), Nω-Nitro-L-arginine methyl ester hydrochloride (100 µM), 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (10 µM), ryanodine (10 µM) and phentolamine (10 µM). SAWF-induced inhibition was mostly restored by cyclopiazonic acid (10 µM). Furthermore, the β-adrenergic receptor antagonist, propranolol (10 µM), abolished SAWF-induced inhibition. These results suggest that SAWF may exert its activity on gastrointestinal smooth muscle via â-adrenergic receptors and sarcoplasmic reticulum Ca2+ ATPase.
Gastric antrum, like other gastrointestinal tissues, exhibits spontaneous motility in the absence of nerve input. This activity is governed by the slow wave - a spontaneous rhythmic depolarization of membrane potential [1]. It is generally accepted that a slow wave is generated by specialized cells called interstitial cells of Cajal (ICC) and these events spread actively within ICC networks and conduct passively to neighboring smooth muscle cells electrically coupled to ICC [2].
Contraction of smooth muscle is regulated by the cytosolic (intracellular) calcium ion (Ca2+) level [Ca2+]i [3,4]. Therefore, the spontaneous contraction of gastrointestinal muscle is affected by endogenous and exogenous substances regulating Ca2+-influx through the membrane or of Ca2+-release from the sarcoplasmic reticulum (SR) [5]. These substances include neurotransmitters such as acetylcholine, adrenalin, and nitric oxide (NO).
Focusing attention on plants medicinally used by indigenous people is the most efficient way to identify herbal plants that may contain bioactive substances. Considering the enormous variety of plant species [6], their potential use as new drug sources has not been completely explored [7,8]. The demand for herbal medicinal products for healthcare is increasing globally [9,10].
Suaeda asparagoides (Miq.) is a halophyte that belongs to the family Chenopodiaceae, which has been used as a traditional Korean folk herbal medicine for the treatment of functional gastrointestinal disorders and is also reported to exhibit anti-oxidative and anti-inflammatory activities [11]. However, its pharmacological activity on gastrointestinal motility is unclear. Therefore, the objective of this study was to determine the effect of S. asparagoides water fraction of the extract (SAWF) on antral motility in vitro.
S. asparagoides reportedly used in Korean folk traditional medicine was collected from the Korean province of Gunsan in 2007. Whole plants were labeled, numbered, and annotated with the date and location of collection and the medicinal use.
As per extraction criteria, the whole aerial plant was used for fractional distillation. To prepare initial extracts for pharmacological testing, one part of the dried plant material was macerated with regular shaking for 72 h in 10 parts of ethanol. The ethanol extract was filtered and evaporated at low temperature (not exceeding 40℃) using a digital rotary evaporator (Tokyo Rikakiki, Tokyo, Japan). The crude extract obtained after ethanol evaporation was successively treated by shaking for 48 h in the following solvents: hexane, chloroform, ethyl acetate, and butanol. Each layer of solvent extract was separated [11]. The final water fraction was then dried in vacuo (BioTron, Gangneung, Korea). The dried extract material (SAWF) was dissolved in distilled water and used for the experiments.
Adult male Sprague-Dawley rats weighing 250-350 g were kept in a group cage under standard environmental conditions in an automatically controlled temperature (21±0.5℃), with 12-h light and dark cycle in a laboratory animal facility. The rats had free access to standard laboratory animal food pellets and water. The investigation conformed to the Guide for the Care and Use of Laboratory Animals protocol, published by the United States National Institutes of Health.
Rats were sacrificed by cervical dislocation and the stomachs were immediately removed and placed in oxygenated (95% O2 and 5% CO2) Krebs-Ringer bicarbonate solution (KRB, pH7.4). Subsequently, each stomach was opened along the lesser curvature and the luminal contents were thoroughly washed using KRB. The mucosa and sub-mucosa were removed by sharp dissection and pinned to the bottom of Sylgard-coated Petri dishes, with the mucosal aspect of the antral muscle facing upward.
Muscle strips (2 mm wide by 8 mm long) were cut along the circular muscle fiber layer of the gastric antrum, suspended in an organ bath containing 10 mL of KRB maintained at 37℃, and bubbled with 95% O2 and 5% CO2. The lower end of each muscle strip was fixed to a stationary holder, and the upper end was attached to a model FT-03 force displacement transducer (Grass-Telefactor, West Warwick, RI, USA) for measuring changes in isometric tension. The signals from the transducer were processed and recorded through Power Lab 2/25 and Chart 5.01 software (AD Instruments, Bella Vista, Australia). Tissues were mounted with an initial load of 0.5 g. After an equilibration period of 60 min with a 10-min wash-out interval, responses of the circular antral muscle to all the treatments were examined. Between treatments, the strips were washed out at least three times every 10 min.
The composition of KRB solution used in this study was (in mM) 118 NaCl, 4.7 KCl2, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 11 dextrose, and 2.5 CaCl2. The pH of the buffer was 7.4 when bubbled with 95% O2 and 5% CO2 at 37℃. Nω-nitro-L-argininemethyl ester (L-NAME), propranolol, phentolamine, and tetrodotoxin were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ), cyclopiazonic acid (CPA), α-adrenergic antagonist receptor (phentolamine), Na+ channel blocker (tetrodotoxin, TTX, 1 µM); sarcoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors, and ryanodine were purchased from Tocris Bioscience (Ellisville, MO, USA). L-NAME, phentolamine, ryanodine, propranolo and TTX were dissolved in distilled water. ODQ and CPA were dissolved in dimethylsulfoxide (DMSO). The final bath concentration of DMSO did not exceed 0.1% v/v.
Values are mean±SEM; n represents the number of animals from which muscle strips were obtained. Paired and unpaired t-tests and one-way ANOVA (repeated measures) followed by multiple comparisons against the control (Tukey's test) were used for statistical comparisons. P<0.05 was used as a cut off for statistical significance in all statistical procedures.
In a preliminary experiment, we examined the effects of extracts of various solvent fractions (ethanol, hexane, chloroform, ethyl acetate, butanol, and water) of S. asparagoides on antral motility. SAWF showed the strongest effects, and was used in the subsequent experiments.
The antral smooth muscle strips showed rhythmic spontaneous phasic contraction. To evaluate the effect of SAWF on the spontaneous contractility of rat gastric antrum in vitro, SAWF was administered in a cumulative method (1-300 mg/mL). Low concentrations (1, 3, and 10 mg/mL) of SAWF increased the amplitude of antral spontaneous phasic contraction, whereas high concentrations (>10 mg/mL) caused a decrease in the motility of antral circular muscle (Figure 1).
To investigate the inhibitory effect of SAWF on the gastric antral contractility, SAWF (100 µg/mL) was administered in the bath in the presence or in the absence of the inhibitors. SAWF induced an immediate and long-lasting inhibition of spontaneous phasic contraction in the circular gastric muscle strips (Figure 2A). The effects of SAWF were fully reversible after wash-out.
The sodium channel blocker TTX at a concentration of 1 µM did not affect the spontaneous phasic contraction of rat gastric antral muscle strips (Figures 2B and 2C). Pretreatment of TTX did not significantly affect the amplitude ratio of the remnant phasic contraction after SAWF administration (Figure 2D).
To determine whether the NO pathway was involved in the inhibitory action of SAWF on gastric antral phasic contraction, L-NAME (a NO synthase inhibitor) and ODQ (an inhibitior of soluble guanylyl cyclase) were administered. Administration of L-NAME (100 µM) evoked statistically insignificant increases of basal tone and amplitude of phasic contraction (Figures 3B and 3C). Pretreatment with L-NAME (100 µM) slightly and statistically insignificantly increased the amplitude ratio of the remnant phasic contraction after SAWF administration (Figure 3D). Pre-incubation with ODQ (10 µM) also increased basal tone and the amplitude of phasic contraction; the increases were not significant (Figures 4B and 4C), and slightly but significantly increased the amplitude ratio of the remnant spontaneous contraction after SAWF (Figure 4D). In the resting state, SAWF did not affect the basal tone of gastric antral muscle (Figures 2A and 3A). However, the increased basal tone state by L-NAME and ODQ was reversed by SAWF to the resting state levels (Figures 3B and 4B).
The inhibitory effect of SAWF was examined with some modulators of intracellular Ca2+ regulation. Pretreatment of antral muscle strips with the SR membrane channel/ryanodine receptor antagonist ryanodine (10 µM) appreciably increased basal tone (Figure 5B). Ryanodine also showed a non-significant increase in the phasic contraction (Figures 5B and 5C). However, ryanodine did not modify the ongoing SAWF inhibitory effect on rat gastric antrum phasic contraction (Figure 5D). On the other hand, the SR Ca2+ATPase inhibitor cyclopiazonic acid (CPA, 10 µM) evoked a transient increase of basal tone and significantly decreased the amplitude of the spontaneous contraction of gastric antral muscle (Figures 6B and 6C). However, CPA mostly protected the amplitude of the phasic contraction from SAWF inhibition (Figure 6D).
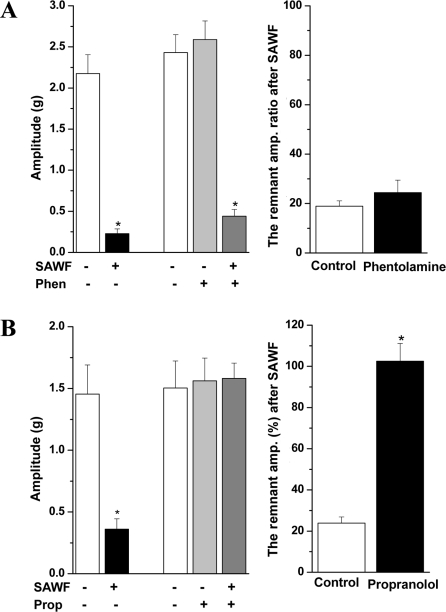
To ascertain the involvement of adrenoceptor in the action of SAWF, the effects of SAWF were evaluated in the absence or in the presence of α- and β-adrenergic receptor antagonists. Pretreatment of muscle strips with the nonselective α-adrenergic receptor blocker phentolamine (10 µM) did not induced significant changes in the amplitude of spontaneous contraction and did not affect the action of SAWF (Figure 7A). However, the β-adrenergic receptor antagonist propranolol (10 µM) did not affect the amplitude of phasic contraction, but completely blocked the inhibitory effect of SAWF (Figure 7B).
Gastric antrum, like other gastrointestinal tissues, exhibits spontaneous motility in the absence of nerve input. This activity is regulated by spontaneous rhythmic depolarization and repolarization of membrane potential, a phenomenon termed slow waves [1], which are generated by ICC [2,12]. In the present study, SAWF exhibited a biphasic dose-response profile in the isolated gastric antral muscle. Lower concentrations of SAWF increased contraction, while higher concentrations produced inhibition. These biphasic effects of herbal extracts have been reported in gastrointestinal motility [13,14], cellular proliferation [15], antihyperglycemic activity [16], and the secretion of interleukin-1β and tumor necrosis factor-α by mononuclear cells [17]. These data suggest that our extract may have both stimulatory and inhibitory components.
Lower concentrations of SAWF may accelerate gastric emptying and contribute to their efficacy in treating symptoms associated with gastroparesis [18]. Stimulating gastric motility agents also enhances gastric emptying [19-21]. On the other hand, higher concentrations SAWF may have a beneficial effect on symptoms associated with rapid gastric emptying. This study, however, focused on the inhibitory mechanism of SAWF.
The inhibitory response exhibited by the extract was not affected by the nerve blocker TTX, suggesting that the observed pharmacological effects are not mediated through the nerves.
It is now widely recognized that NO is an important physiological mediator of nonadrenergic-noncholinergic (NANC) relaxation of gastrointestinal smooth muscle [22,23]. NO is synthesized by NO synthase and NO increases the intracellular cGMP concentration through activation of soluble guanylate cyclase, and cGMP induces various cellular responses via protein kinase G-dependent phosphorylation of intracellular target proteins including ion channels [24].
In this study, the NO synthase inhibitor L-NAME showed a tendency to slightly and statistically insignificantly reduce the effects of SAWF on antral circular muscle motility. The soluble guanylate cyclase inhibitor ODQ significantly suppressed SAWF effects on the amplitude of antral spontaneous contraction. These data suggest that the SAWF effect on antral motility might involve the NO pathway but the portion of NO pathway is very small.
It is generally accepted that regulation of intracellular calcium concentration ([Ca2+]i) is important in pacemaking of the gastrointestinal tract [25,26], and in the contraction and relaxation of smooth muscle [25,26]. Ryanodine, which is a SR receptor antagonist, is frequently used to deplete the SR by causing the Ca2+ release channels to remain in a semi-conducting state, resulting in an increased cytosolic Ca2+ concentration [27].
In the present study, ryanodine increased the basal tone and amplitude of phasic contraction in gastric antral muscle. These ryanodine effects might be due to increased [Ca2+]i. However, ryanodine did not affect the SAWF induced inhibition, suggesting that the extract effect may not be mediated through the ryanodine receptor.
Laporte et al [27] and Thorneloe and Nelson [5] indicated that Ca2+ removal in smooth muscle is critically important to mediate smooth muscle relaxation and maintain Ca2+ homeostasis. Ca2+ removal occurs by means of uptake of Ca2+ into the SR via Ca2+-ATPase (SERCA pump).
In the present study, CPA transiently increased the basal tone and decreased amplitude of spontaneous contraction in gastric antral muscle. However, CPA protected the large part of the amplitude in the antral phasic contraction from SAWF. Therefore, it can be concluded that acceleration of the SR Ca2+-ATPase (SERCA) activity and SR Ca2+ uptake may play a greater role in the inhibitory action of SAWF on gastric motility.
The sympathetic nervous system has an inhibitory effect on gastrointestinal motility [28]. The inhibitory effect of sympathetic nerve stimulation in the gastrointestinal tract is mediated by the activation of postjunctional β-adrenoceptors (β-AR) and all three β-AR subtypes, β1-, β2- and β3-AR, exist in the gastrointestinal tract [29,30].
In the present study, the sustained inhibition of the spontaneous phasic contraction by SAWF in the rat gastric antrum was unaffected by the α-adrenergic receptor antagonist, phentolamine, but was abolished by the β-adrenergic receptor antagonist, propranolol. These data indicate that the inhibition of spontaneous contractile activity by SAWF is mediated by β-adrenergic pathways.
It is generally accepted that activation of β-AR evokes accumulation of cAMP then activates cAMP-dependent protein kinase A ([27,31]. Phospholamban regulates the contractility of smooth muscle by reducing Ca2+ uptake into the SR. When phospholamban is phosphorylated by protein kinase A, SERCA activity is increased, resulting in an enhanced uptake of Ca2+ by the SR and reduction of [Ca2+]i and subsequent muscle relaxation [4,5,32].
In conclusion, the water fraction of S. asparagoides extract (SAWF) inhibits rat gastric antral phasic contraction. This inhibitory effect of SAWF is completely blocked by propranolol (β-adrenoceptor antagonist) and almost abolished by CPA (SR Ca2+-ATPase inhibitor). These data suggest that the inhibitory action of SAWF on the gastric antral motility is mediated by β-adrenoceptor and is mostly performed by accelerating the SR Ca2+-ATPase.
References
1. Szurszewski JH. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978; 277:91–102. PMID: 650594.

2. Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68:307–343. PMID: 16460275.

3. Bolton TB. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J Physiol. 2006; 570(Pt 1):5–11. PMID: 16195319.

4. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997; 49(2):157–230. PMID: 9228665.
5. Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005; 83(3):215–242. PMID: 15870837.

6. Gadano AB, Gurni AA, Carballo MA. Argentine folk medicine: genotoxic effects of Chenopodiaceae family. J Ethnopharmacol. 2006; 103(2):246–251. PMID: 16219440.
7. Akah PA, Aguwa CN, Agu RU. Studies on the antidiarrhoeal properties of Pentaclethra macrophylla leaf extracts. Phytother Res. 1999; 13(4):292–295. PMID: 10404533.
8. Amos S, Binda L, Kunle OF, Okafor I, Emeje M, Akah PA, Wambebe C, Gamaniel K. Smooth muscle contraction induced by Indigofera dendroides leaf extracts may involve calcium mobilization via potential sensitive channels. Phytother Res. 2003; 17(7):792–796. PMID: 12916079.
9. Hohenester B, Ruhl A, Kelber O, Schemann M. The herbal preparation STW5 (lberogast) has potent and region-specific effects on gastric motility. Neurogastroenterol Motil. 2004; 16(6):765–773. PMID: 15601427.
10. Roman-Ramos R, Flores-Saenz JL, Alarcon-Aguilar FJ. Anti-hyperglycemic effect of some edible plants. J Ethnopharmacol. 1995; 48(1):25–32. PMID: 8569244.

11. Park JM, Kim SD, Lee WM, Cho JY, Park HJ, Kim TW, Choe NH, Kim SK, Rhee MH. In vitro anti-oxidative and antiinflammatory effects of solvent-extracted fractions from Suaeda asparagoides. Pharmazie. 2007; 62(6):453–458. PMID: 17663194.
12. Kim TW, Beckett EA, Hanna R, Koh SD, Ordog T, Ward SM, Sanders KM. Regulation of pacemaker frequency in the murine gastric antrum. J Physiol. 2002; 538(Pt 1):145–157. PMID: 11773323.

13. Amos S, Binda L, Chindo B, Akah P, Abdurahman M, Danmallam HU, Wambebe C, Gamaniel K. Evaluation of methanolic extract of Ficus platyphylla on gastrointestinal activity. Indian J Exp Biol. 2001; 39(1):63–67. PMID: 11349528.
14. Gilani AH, Aziz N, Ali SM, Saeed M. Pharmacological basis for the use of peach leaves in constipation. J Ethnopharmacol. 2000; 73(1-2):87–93. PMID: 11025143.

15. Liu YH, Li ML, Hsu MY, Pang YY, Chen IL, Chen CK, Tang SW, Lin HY, Lin JY. Effects of a Chinese herbal medicine, Guan-Jen-Huang (Aeginetia indica Linn.), on renal cancer cell growth and metastasis. Evid Based Complement Alternat Med. 2012; in press.
16. Anaga AO, Njoku CJ, Ekejiuba ES, Esiaka MN, Asuzu IU. Investigations of the methanolic leaf extract of Costus afer. Ker for pharmacological activities in vitro and in vivo. Phytomedicine. 2004; 11(2-3):242–248. PMID: 15070179.
17. Chang JY, Yang TY, Chang CP, Chang JG. The effect of "chi-han (hot nature)" Chinese herbs on the secretion of IL-1β and TNF-α by mononuclear cells. Kaohsiung J Med Sci. 1996; 12(1):18–24. PMID: 8871284.
18. Galligan JJ, Vanner S. Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil. 2005; 17(5):643–653. PMID: 16185302.

19. Wang Y, Kondo T, Suzukamo Y, Oouchida Y, S . Izumi. Vagal nerve regulation is essential for the increase in gastric motility in response to mild exercise. Tohoku J Exp Med. 2010; 222(2):155–163. PMID: 20948179.
20. Kawachi M, Matsunaga Y, Tanaka T, Hori Y, Ito K, Nagahama K, Ozaki T, Inoue N, Toda R, Yoshii K, Hirayama M, Kawabata Y, Takei M. Acotiamide hydrochloride (Z-338) enhances gastric motility and emptying by inhibiting acetylcholinesterase activity in rats. Eur J Pharmacol. 2011; 666(1-3):218–225. PMID: 21651906.

21. Toyomasu Y, Mochiki E, Yanai M, Ogata K, Tabe Y, Ando H, Ohno T, Aihara R, Zai H, Kuwano H. Intragastric monosodium L-glutamate stimulates motility of upper gut via vagus nerve in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R1125–R1135. PMID: 20071606.
22. Kim T, La J, Lee J, Yang I. Effects of nitric oxide on slow waves and spontaneous contraction of guinea pig gastric antral circular muscle. J Pharmacol Sci. 2003; 92(4):337–347. PMID: 12939518.

23. Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992; 262(3 Pt 1):G379–G392. PMID: 1347974.

24. Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993; 1178(2):153–175. PMID: 7688574.

25. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000; 525(Pt 2):355–361. PMID: 10835039.

26. Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl- conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009; 587(Pt 20):4905–4918. PMID: 19703958.
27. Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004; 56(4):439–513. PMID: 15602008.

28. Bojo L, Nellgard P, Cassuto J. Effects of selective adrenergic agonists and antagonists on gastric tone in the rat. Acta Physiol Scand. 1991; 142(4):517–522. PMID: 1683093.
29. Manara L, Croci T, Aureggi G, Guagnini F, Maffrand JP, Le Fur G, Mukenge S, Ferla G. Functional assessment of â adrenoceptor subtypes in human colonic circular and longitudinal (taenia coli) smooth muscle. Gut. 2000; 47(3):337–342. PMID: 10940268.
30. Roberts SJ, Papaioannou M, Evans BA, Summers RJ. Functional and molecular evidence for β1-, β2- and β-3-adrenoceptors in human colon. Br J Pharmacol. 1997; 120(8):1527–1535. PMID: 9113375.

31. Tanaka Y, Horinouchi T, Koike K. New insights into β-adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin Exp Pharmacol Physiol. 2005; 32(7):503–514. PMID: 16026507.

32. Colyer J. Phosphorylation states of phospholamban. Ann N Y Acad Sci. 1998; 853:79–91. PMID: 10603938.
Figure 1
Biphasic effects of SAWF on the spontaneous contractility of rat gastric antral circular muscle. Low concentrations (1-10 mg/mL) of SAWF increased the motility of gastric antral circular muscle, whereas high concentrations (>10 mg/mL) repressed motility.
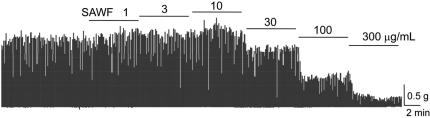
Figure 2
Effect of Na+ channel blocker, tetrodotoxin (TTX), on SAWF-induced responses of strips from circular muscles of rat gastric antrum. (A and B) Antral smooth muscle strip responses induced by SAWF (100 µg/mL) alone (A) or in the presence of TTX (1 µM) (B). (C) This experiment performed in same strip. Actual values for amplitude inhibition effect of SAWF alone and TTX pretreated antral muscle strips. (D) Pretreatment of TTX did not significant affect on the amplitude ratio of the remnant phasic contraction after SAWF administration. Values are mean±SEM. *Significantly different from previous column, P<0.05 (n=6).
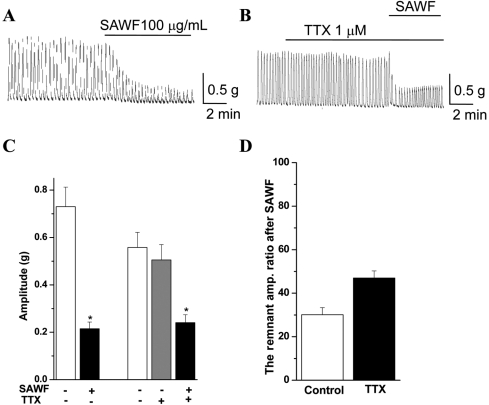
Figure 3
Effect of L-NAME on SAWF evokes relaxation of rat gastric antrum. (A) SAWF-induced inhibition on antral spontaneous phasic contraction. (B) Panel for the effect of SAWF on L-NAME pretreated antral muscle strip. (C) Summary for the actual values of control, SAWF and L-NAME treatment groups. (D) Pretreatment of L-NAME (100 µM) showed a slight tendency to increase the amplitude ratio of the remnant phasic contraction after SAWF administration without significance. Values are mean±SEM. *Significantly different from previous column, P<0.05 (n=6).
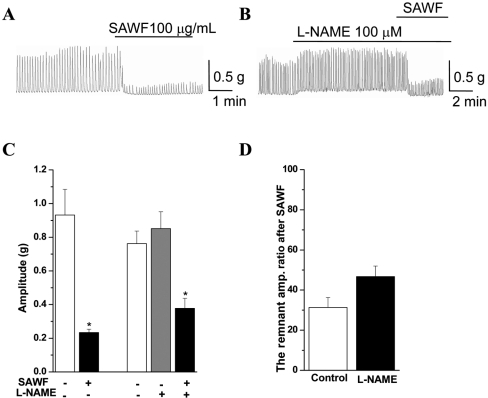
Figure 4
Effect of ODQ on SAWF induced inhibition on amplitude of rat gastric antral muscle phasic contraction. (A) Panel for SAWF-induced inhibition on the amplitude of rat antral spontaneous phasic contraction (B) Panel for the effect of SAWF on ODQ pretreated antral muscle strip. (C) Summary for the actual value of control, SAWF, and ODQ treatment groups. (D) ODQ (10 µM) slightly but significantly increased the amplitude ratio of the remnant spontaneous contraction after SAWF administration. Values are mean±SEM. *Significantly different from previous column, P<0.05 (n=6).
Figure 5
Effect of ryanodine on SAWF induced inhibition on amplitude of rat gastric antral muscle phasic contraction. (A) Panel for SAWF induced inhibition on the amplitude of spontaneous phasic contraction (B) Panel for the effect of SAWF on ryanodine pretreated antral muscle strip. The bars over the panels indicate the duration of administration of the extract ryanodine. (C) Summary for the control, SAWF and ryanidine treatment groups. (D) Ryanodine (10 µM) did not affect SAWF induced inhibition ratio on the amplitude of rat antral muscle phasic contraction. Values are mean±SEM. *Significantly different from previous column, P<0.05 (n=6).
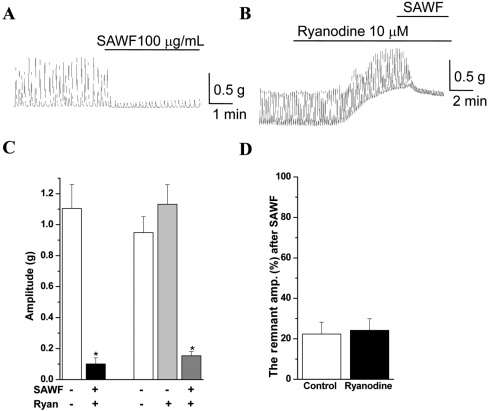
Figure 6
Effects of CPA on SAWF evoked inhibition of antral muscle phasic contraction. (A) Panel for SAWF-induced inhibition on the amplitude of spontaneous phasic contraction. (B) Sarcoplasmic reticulum Ca2+-ATPase inhibitor, cyclopiazonic acid (CPA, 10 µM), did not affect on the gastric antral motiltity. (C) Actual values on SAWF alone & in the presence of CPA with their respective controls. (D) CPA mostly protected the amplitude of the phasic contraction from SAWF inhibition. Values are mean±SEM. *Significantly different from previous column, P<0.05 (n=6).
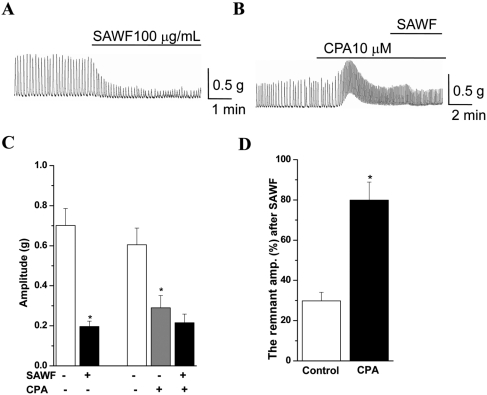
Figure 7
Effects of α- and β-adrenergic receptor antagonists on SAWF induced inhibition. (A) Phentolamine, α-adrenergic receptor (10 µM) did not affect SAWF evoked inhibition of rat antral muscle phasic contraction. (B) However, propranolol, a β-adrenergic receptor antagonist (10 µM), completely abolished SAWF induced inhibition of rat antral muscle phasic contraction (B) Values are mean±SEM. *Significantly different from previous column, P<0.05 (n=6).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download