Abstract
Renal toxicity by melamine in combination with cyanuric acid (1:1) was investigated. Male rats were orally administered melamine plus cyanuric acid (5, 50 or 400 mg/kg each) for 3 days. In contrast to a negligible effect by melamine alone (50 mg/kg, a no-observed-adverse-effect-level: NOAEL), co-administration with cyanuric acid markedly increased the concentrations of blood urea nitrogen and creatinine, as well as kidney weight. A high dose (400 mg/kg) of melamine plus cyanuric acid induced more severe kidney toxicity. The increased blood parameters for kidney toxicity and organ weight lasted longer than 4 days. Combined treatment with melamine and cyanuric acid (50-400 mg/kg each) resulted in many gold-brown crystals and toxic lesions in renal tubules, which were not observed in animals treated with melamine alone (50 mg/kg). These results indicate that only a 3-day exposure to melamine in combination with cyanuric acid causes severe renal damage, even at a NOAEL for melamine found in a 13-week toxicity study. Therefore, it is suggested that the tolerable daily intake or regulatory/management levels of melamine need to be re-considered for cases of co-exposure with cyanuric acid.
Numerous dogs and cats were poisoned, and many died after consumption of pet feeds adulterated with gluten that was contaminated by melamine in the USA and Korea in 2004 and 2007 (Brown et al, 2007). More importantly, about 294,000 children suffered from renal dysfunctions after consuming milk powder contaminated with melamine, and 6 children died in China in 2008 (Ingelfinger, 2008; WHO, 2008).
Melamine analogues, such as ammeline, ammelide and cyanuric acids, are formed as byproducts during the process of melamine production or by bacterial degradation (Cook et al, 1985). However, melamine and cyanuric acid are not metabolized by mammals (Ames et al, 1979; Hammond et al, 1986). Gluten, a source for the outbreak of animal poisonings, was shown to contain 8.4% melamine, 5.3% cyanuric acid, 2.3% ammelide, 1.7% ammeline and other melamine analogues (<1%) (Dobson et al, 2008). Melamine and cyanuric acid are thought to form a crystal structure after they bind with each other (Puschner et al, 2007). Accordingly, Dobson et al (2008) demonstrated that renal calculi and tubular damage could occur when rats were fed 3-day combined melamine and cyanuric acid at 400 mg/kg each. These doses were adapted from the level of contamination in pet diet that had induced the same toxicities in cats.
The no-observed adverse-effect level (NOAEL) of melamine was originally found to be 63 mg/kg based on the induction of renal toxicity (bladder calculi) in a 13-week toxicity study using rats treated with melamine alone (OECD, 1998). Thus, a tolerable daily intake (TDI) for humans was determined to be 0.5-0.63 mg/kg, with a safety factor of 100, in Europe and the USA, although the TDI was recently reduced by an expert group at the 2008 WHO meeting to 0.2 mg/kg (WHO, 2008). Cyanuric acid also did not induce marked renal toxicity up to 150 mg/kg in rodents, leading to 1.5 mg/kg of TDI (Hammond et al, 1986; OECD, 1999). Against this background, we examined if kidney toxicity could be induced by the treatment of melamine in combination with cyanuric acid to rats at NOAEL and contamination levels in milk powder or animal feed, compared to the toxicity induced by melamine only.
Seven-week-old male Sprague-Dawley rats were purchased from the Orient-Bio Co. (Seongnam, Korea). The animals were housed at the Laboratory Animal Research Center of Chungbuk National University, Korea. The animals were maintained in a room with constant temperature of 22±1℃, relative humidity of 55±10%, and 12-h light/dark cycle, and fed standard rodent chow (Samyang Co., Gapyeong, Korea) and purified tap water ad libitum.
After 1-week acclimation to the laboratory environments, the animals were orally administered melamine (Sigma, St. Louis, USA) alone or in combination with cyanuric acid (Sigma) for 3 days. These were dissolved in carboxymethylcellulose (1%) at a ratio of 1:1 according to the stoichiometric reaction (Puschner et al, 2007; Dobson et al, 2008). The rats were divided into several groups (n=6 per group): 1) the highest contamination level in pet diet (8,000 ppm≒400 mg/kg each melamine and cyanuric acid), 2) the NOAEL (50 mg/kg each) and 3) the mean contamination level in food (100 ppm≒5mg/kg each). Additional groups were allocated for comparisons with the toxicity by melamine only (50 mg/kg melamine), and for examining the recovery from nephrotoxicity following withdrawal of the treatment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Chungbuk National University.
Twenty-four hours after the final treatment, the rats were sacrificed for the measurements of kidney weights and for gross and microscopic examinations. Separately, several animals (n=6) treated with 50 or 400 mg/kg of melamine and cyanuric acid were examined for their recoveries 4 days after the last treatment. Formalin-fixed, paraffin-embedded tissue sections (4 µm in thickness) were stained with hematoxylin and eosin, and examined under a light microscope.
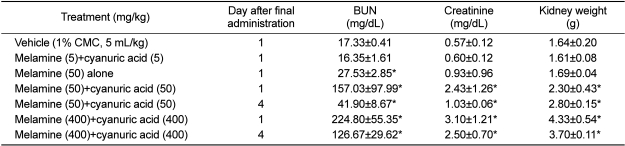
Based on the serum biochemistry and kidney weights, it appeared that a low dose (5 mg/kg) of melamine plus cyanuric acid did not induce renal toxicity (Table 1). However, the rats administered 50 mg/kg of melamine plus cyanuric acid showed marked increases in the levels of blood urea nitrogen (BUN) and creatinine as well as their kidney weights. By comparison, the animals treated with melamine alone (50 mg/kg, a NOAEL) showed only a slight increase in BUN, with no effects on the creatinine level or kidney weights. The increased BUN and creatinine levels induced by the combined treatment with melamine and cyanuric acid (50 mg/kg each) were remarkably decreased, but still remained statistically higher than controls after 4 days of withdrawal. However, the kidney weight did not recover. A higher dose (400 mg/kg) of melamine plus cyanuric acid induced more severe kidney toxicity, for which the increased blood parameters and kidney weights lasted longer than 4 days.
On gross findings, no changes were observed in the animals treated with 5 mg/kg of melamine plus cyanuric acid or 50 mg/kg of melamine alone (Figure 1). However, the rats treated with the combination of melamine and cyanuric acid (50 mg/kg each) showed enlargements of the kidneys with gold-brown colors. These changes (swelling and discoloration) were much more severe in animals treated with 400 mg/kg melamine plus cyanuric acid.
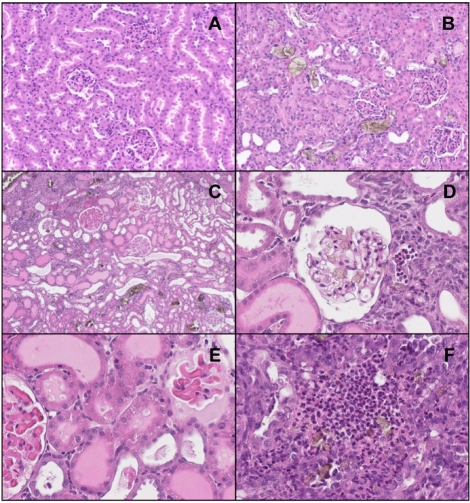
Histopathologically, no toxic lesions were observed in the animals treated with 5 mg/kg of melamine plus cyanuric acid or 50 mg/kg of melamine alone. By comparison, many bluish brown crystals were found in the distal tubules and collecting ducts of the rats treated with the combination of melamine and cyanuric acid (50 mg/kg each) (Figure 2). In addition to these lesions, eosinophilic, amorphous, proteinaceous casts filled in the severely-dilated renal tubules in the kidneys of high dose rats of melamine plus cyanuric acid (400 mg/kg each). These crystals were present even in atrophic glomeruli. Numerous hyaline droplets accumulated in the epithelial cells of proximal tubules. It is of interest to note that acute tubulointerstitial nephritis, characterized by severe infiltration by neutrophils in the tubular lumen and interstitium, and fibrosis occurred.
Since triazines including melamine and cyanuric acid are not cytotoxic to kidney cells, it is proposed that the nephrotoxicity results initially from a physical blockage by crystals (Dobson et al, 2008). In addition, there may be critical points in the concentrations of melamine and cyanuric acid to form insoluble crystals by producing multiple hydrogen bonds under specific environments of the kidneys (Whitesides et al, 1991; Dobson et al, 2008). Virtually, no crystals and lesions were observed in rats treated with 5 mg/kg of melamine plus cyanuric acid when compared to the animals co-administered 50 mg/kg each (the present study) or 400 mg/kg and 40 mg/kg of melamine and cyanuric acid, respectively, which showed many crystals and marked nephrotoxicity (Dobson et al, 2008). Several studies reported that melamine can be rapidly eliminated to the urine without metabolism in three hours of half-life (OECD, 1998), and that melamine has diuretic activity (Brown et al, 2007; Dobson et al, 2008). Therefore, it seems that a low dose (5 mg/kg) of melamine in combination with cyanuric acid would be easily cleared with limited formation and accumulation of crystals prior to the next (in 24 hours) daily treatment.
Dobson et al (2008) reported profiles of renal crystals in rats treated with relatively high doses of melamine and their byproducts without in-depth explanation on the features of the crystals and their distribution in the kidneys. However, more detailed nature of the crystals was shown in dogs and cats poisoned following consumption of pet feeds adulterated with gluten containing melamine and cyanuric acid (Brown et al, 2007). Such findings were experimentally confirmed in cats by feeding a diet containing both melamine and cyanuric acid (Puschner et al, 2007). Typically, fan-shaped, bluish brown melamine crystals were found in distal tubules and collecting ducts of the kidneys, whereas oblong, lighter green, glassy oxalate crystals were found in proximal tubules induced by ethylene glycol. Unlike the proximal tubular injuries by oxalate-forming toxicants such as ethylene glycol, ochratoxin and citrinin, the distribution of crystals and nephritis in distal parts of the kidneys are one of the distinct features of melamine toxicity (Kitchen et al, 1977; Kogika et al, 1993; Brown et al, 2007). However, a high dose (400 mg/kg) of melamine plus cyanuric acid caused more wide crystal distributions, even in glomeruli (Figure 2D), which resulted in extensive lesions including proteinaceous casts in dilated distal tubules, hyaline droplets in proximal tubules, glomerular atrophy, and inflammatory responses in tubular lumen and interstitium (Figures 2C-2F). Furthermore, in spite of the recovery from the highly-increased BUN and creatinine levels 4 days after the withdrawal, the kidney weights and lesions were not readily improved. This may be due to the continuous irritation by long-lasting insoluble crystals as shown in a previous study performed in dogs and cats (Brown et al, 2007).
In summary, these results suggest that oral ingestion of melamine in combination with cyanuric acid causes more severe renal damage than that by melamine alone. Notably, co-administration of melamine and cyanuric acid for only 3 days apparently induced nephrotoxicity even at a NOAEL (50 mg/kg) for melamine. In this light, we concluded that TDI or regulatory/management levels of melamine need to be re-considered for cases of co-exposure with cyanuric acid.
Acknowledgment
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094035).
References
1. Ames MM, Powis G, Kovach JS, Eagan RT. Disposition and metabolism of pentamethylmelamine and hexamethylmelamine in rabbits and humans. Cancer Res. 1979; 39:5016–5021. PMID: 115586.
2. Brown CA, Jeong KS, Poppenga RH, Puschner B, Miller DM, Ellis AE, Kang KI, Sum S, Cistola AM, Brown SA. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J Vet Diagn Invest. 2007; 19:525–531. PMID: 17823396.

3. Cook AM, Beilstein P, Grossenbacher H, Hütter R. Ring cleavage and degradative pathway of cyanuric acid in bacteria. Biochem J. 1985; 231:25–30. PMID: 3904735.

4. Dobson RLM, Motlagh S, Quijano M, Cambron RT, Baker TR, Pullen AM, Regg BT, Bigalow-Kern AS, Vennard T, Fix A, Reimschussel R, Overmann G, Shan Y, Daston GP. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol Sci. 2008; 106:251–262. PMID: 18689873.

5. Hammond BG, Barbee SJ, Inoue T, Ishida N, Levinskas GJ, Stevens MW, Wheeler AG, Cascieri T. A review of toxicology studies on cyanurate and its chlorinated derivatives. Environ Health Perspect. 1986; 69:287–292. PMID: 3545805.

6. Ingelfinger JR. Melamine and the global implications of food contamination. N Engl J Med. 2008; 359:2745–2748. PMID: 19109571.

7. Kitchen DN, Carlton WW, Tuite J. Ochratoxin A and citrinin induced nephrosis in Beagle dogs. II. Pathology. Vet Pathol. 1977; 14:261–272. PMID: 883089.

8. Kogika MM, Hagiwara MK, Mirandola RM. Experimental citrinin nephrotoxicosis in dogs: renal function evaluation. Vet Hum Toxicol. 1993; 35:136–140. PMID: 8470356.
9. Screening information data set for melamine, CAS No. 108-78-1. OECD. 1998. http://www.chem.unep.ch/irptc/sids/OECDSIDS/108781.pdf.
10. Screening information data set for isocyanuric acid, CAS No. 108-80-5. OECD. 1999. http://www.chem.unep.ch/irptc/sids/OECDSIDS/108805.pdf.
11. Puschner B, Poppenga RH, Lowenstine LJ, Filigenzi MS, Pesavento PA. Assessment of melamine and cyanuric acid toxicity in cats. J Vet Diagn Invest. 2007; 19:616–624. PMID: 17998549.

12. Expert meeting to review toxicological aspects of melamine and cyanuric acid. Executive summary. WHO. 2008. http://www.who.int/foodsafety/fsmanagement/ExecSummarymelamine.pdf.
13. Whitesides GM, Mathias JP, Seto CT. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science. 1991; 254:1312–1319. PMID: 1962191.

Figure 1
Gross findings of the kidneys of rats given oral melamine (M) alone or in combination with cyanuric acid (C) for 3 days. Control, 1% carboxymethylcellulose (5 mL/kg); M5+C5, 5 mg/kg melamine+5 mg/kg cyanuric acid; M50, 50 mg/kg melamine; M50+C50, 50 mg/kg melamine+50 mg/kg cyanuric acid; M400+C400, 400 mg/kg melamine+400 mg/kg cyanuric acid.
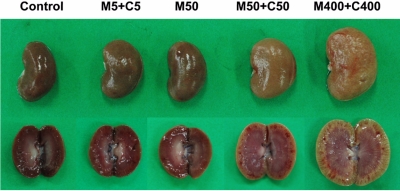
Figure 2
Microscopic findings for the kidneys of rats given oral melamine plus cyanuric acid for 3 days. A, 50 mg/kg melamine alone, exhibiting normal features; B, 50 mg/kg melamine+50 mg/kg cyanuric acid, displaying many bluish brown crystals in distal tubules and collecting ducts with focal dilatation; C-F, 400 mg/kg melamine+400 mg/kg cyanuric acid, showing crystals in severely-dilated tubules filled with proteinaceous casts (C) and in an atrophic glomerulus (D), hyaline droplets in the epithelial cells of proximal tubules (E) and neutrophil infiltrations in tubular lumen and interstitium (F).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download