1. Cho YJ, An BJ, Kim MU, Shim CS. Anti-inflammatory effect of Aloe vera and aloe arborescens in phosphatidic acid-stimulated raw cells. J Korean Soc Appl Biol Chem. 2006; 49(1):65–69.
2. Kang MH, Cho SY, Kim HS, Kim DH, Jeong CS. Antigastritic and antiulcerative effect of Pulmuone Healthy Aloe Gel. Yakhak Hoeji. 2005; 49(3):237–243.
3. Hong HS, Lee KH, Kim JH, Kang HG, Cho CH, Kim CH. The Comparative of inhibitory effect of various solvent extracts from Aloe arborescens and Aloe vera on tumor cell lines using clonoginic assay. Korean J Pharmacogn. 1999; 30(3):275–279.
4. Lee YW, Roh WS, Kim JG, Kim PG. Effects of Aloe vera on the cholesterol and vitamin D2-induced atherosclerosis in rats. J Food Hyg Saf. 1996; 11(4):243–259.
5. Woo N, Ahn MS, Lee SY. Antioxidative Effect of Aloe (Aloe arborescences) Extracts on Linoleic Acid and Soybean Oil. Korean J Soc Food Sci. 1995; 11(5):536–541.
6. Jeong HY, Kim JH, Hwang SJ, Rhee DK. Anticancer effects of aloe on sarcoma 180 in ICR mouse and on human cancer cell lines. Yakhak Hoeji. 1994; 38(3):311–321.
7. Lee GD, Chang HG, Kim HK. Antioxidative and nitrite-scavenging activities of edible mushrooms. Korean J Food Sci Technol. 1997; 29(3):432–436.
8. Lee BW, Lee MS, Park KM, Kim CH, Ahn PU, Choi CU. Anticancer activities of the extract from the mycelia of Coriolus versicolor. Korean J Appl Microbiol Bioeng. 1992; 20(3):311–315.
9. Lee SY, Kang TS, Moon SO, Lew ID, Lee MY. Fractionation and antitumor activity of the water soluble exo-polysaccharide by submerged cultivation of Ganoderma lucidum mycelium. Korean J Appl Microbiol Bioeng. 1996; 24(4):459–464.
10. Kim SW, Kim ES, Kim YS. Studies on the polysaccharide extracted from Ganoderma lucidum. J Korean Soc Food Nutr. 1995; 24(1):147–153.
11. Kim YI, Kim BK, Jeong H, Lee KH. Production of Antihypertensive constituents from Ganoderma lucidum IY005 by fermentation using industrial wastes. Korean J Mycol. 1991; 19(1):79–84.
12. Hwang KH, Kim HK, Han YN. Screening of inhibitory activity of edible mushrooms on dopamine β-hydroxylase. Korean J Food Sci Technol. 1997; 29(2):194–197.
13. Kim SH, Lee JN, Kim SH, Oh SJ, An SW, Lee JH, Park YS, Chung EK, Lee HY. Studies on screening and comparison of biological activities from the fruiting body and mycelium of Elfvingia applanata. Korean J Appl Microbiol Bioeng. 1998; 26(4):331–337.
14. Oh SI, Lee MS. Antioxidative and antimutagenic effects of Ganoderma lucidum Krast extracts. Korean J Food Nutr. 2005; 18(1):54–62.
15. Seo KW, Cho IS, Oh MH, Lee KM, Kim HJ. Subacute toxicity of G009, a polysaccharide isolated from Ganoderma lucidum IY009. J Food Hyg Saf. 1996; 11(4):261–271.
16. Bae HS, Kang SK, Shin IS, Woo SK, Kim YJ, Kim MA, Ra JC. The effects of extracts mixture drink from Inonotus obliquus, Phellinus linteus and Ganoderma lucidum on hematopoietic stem cells and lymphocyte subset of blood in human. J Food Hyg Saf. 2009; 24(1):78–85.
17. Joung EM, Hwang IG, Lee HY, Jeong JH, Yu KW, Jeong HS. Changes of saponin and β-glucan content on the cultured ginseng with mushroom mycelia. J Korean Soc Food Sci Nutr. 2009; 38(8):1084–1089.

18. Jeong JH, Wee JJ, Shin JY, Cho JH, Jung DH. Antioxidative effect of crude saponin fraction prepared from culture product of Basidiomycota cultured with fresh ginseng as substrate. Korean J Food Sci Technol. 2005; 37(1):67–72.
19. Choi MA, Park NY, Woo SM, Jeong YJ, Shin SR. Characteristics of Hericium erinaceus and its extracts. Korean J Food Preserv. 2003; 10(4):560–564.
20. Jung JH, Lee KE, Lee SY. Optimization of submerged cultivation of Hericium erinaceum. Korean J Biotechnol Bioeng. 2006; 21(2):96–102.
21. Park JS, Chang KW, Nam YG. Analysis of barbaloin in the Aloe vera depending on the various extracting conditions. J Korean Soc Appl Biol Chem. 1994; 37(5):409–413.
22. Chang KW, Park JS, Jang GC. Fatty and organic acids, and barbaloin in various parts of Aloe species dried at different drying temperatures. J Korean Soc Appl Biol Chem. 1993; 36(4):244–248.
23. Um S, Jin GE, Park KW, Yu YB, Park KM. Physiological activity and nutritional composition of Pleurotus species. Korean J Food Sci Technol. 2010; 42(1):90–96.
24. Sung IJ, Park MY, Kim JD. Mouse single oral dose toxicity test and bone marrow micronucleus test of Mahwangbujaseshin-tang extracts. Korean J Orient Physiol Pathol. 2010; 24(1):124–133.
25. Jung WC, Lee JH, Choi WJ, Kim S, Lee HJ. Four-week repeated-dose toxicity study of sodium chlorate in mice. Lab Anim Res. 2009; 25(4):355–362.
26. Kim TM, Ryu JM, Seo IK, Yeon S, Lim WT, Lee JY, Hwang SY, Kim YB. Four-week repeated-dose toxicity of silk amino acids in rats. Lab Anim Res. 2008; 24(4):565–573.
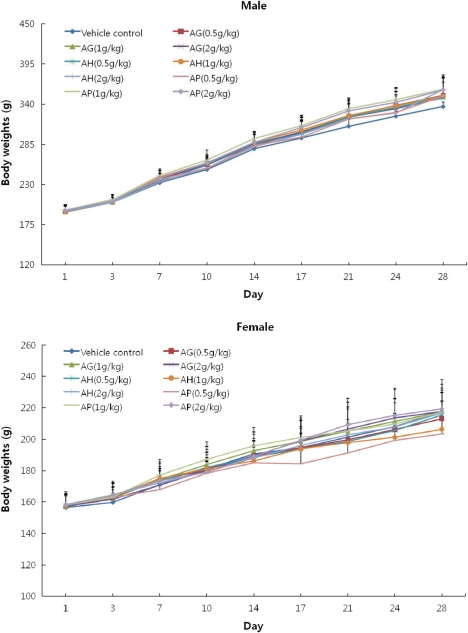
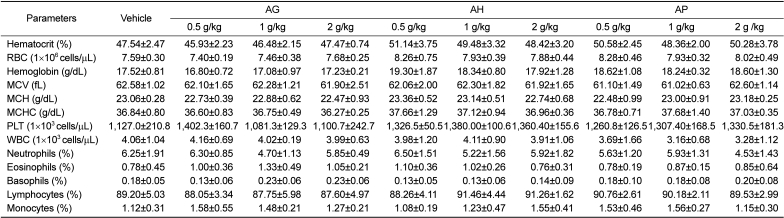
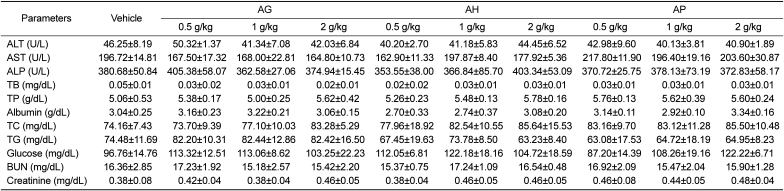
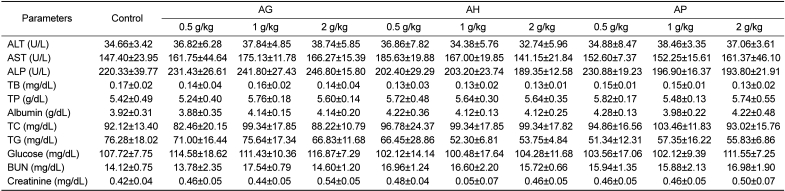




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download