Abstract
IH-901 (20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol or compound K) is a final intestinal bacterial metabolite of ginseng in humans. It has various pharmacologic effects such as antiaging, immunopotentiation, antistress, and antimetastatic activities. We analyzed the antioxidant activities of IH-901 using several assays including: total antioxidant activity, reductive potential, 1,1-diphenyl-2-picryl-hydrazyl, hydroxyl, superoxide and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assays, a nitric oxide scavenging assay and a lipid peroxidation assay. At concentrations of 25 and 100 µg/mL, IH-901 inhibited lipid peroxidation of a linoleic acid emulsion with a potency comparable to ascorbic acid and butylated hydroxyanisole. The reductive potential of IH-901 increased in a concentration-dependent manner. IH-901 exhibited strong DPPH, hydroxyl, superoxide and ABTS radical scavenging activities. IH-901 was also an effective inhibitor of lipid peroxidation, although IH-901 had only a mild scavenging activity against nitric oxide. These results suggest that IH-901 may be a useful antioxidant agent against reactive oxygen species.
Oxidative stress is an important contributor to the pathophysiology of various pathological conditions including cardiovascular dysfunction, atherosclerosis, inflammation, carcinogenesis, drug toxicity, reperfusion injury and neuro-degenerative diseases [1]. Reactive oxygen species (ROS), including free radicals such as superoxide anion radical (O2-), hydroxyl radical (OH-) and the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, are active oxygen compounds that are often generated by biological oxidation reactions with exogenous factors [2]. ROS are known to cause aging and cancer and to have many other toxic effects in the human body. There are many antioxidants that minimize the actions of ROS [3]. Antioxidants have been defined as substances that prevent the formation of ROS or other oxidants, scavenge them, or repair the damage they cause [4]. To date, there have been many reports that ginseng and ginseng derivates have antioxidant properties [3,5,6].
Ginseng has been used frequently as a traditional drug in East Asian countries. Ginseng intestinal metabolite-I (IH-901; 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol or compound K) is a final intestinal bacterial metabolite of ginseng in humans, and has various pharmacologic effects such as antiaging, immunopotentiation, antistress, antimetastatic and antioxidant activities [7-10]. We previously reported that administration of IH-901 ameliorated doxorubicin-induced decreases in phospholipid hydroperoxide glutathione peroxidase (PHGPx) production in mice [11]. Other ginsenoside derivatives such as ginsenoside Rb3 and ginsenoside Rd were also reported to have antioxidative effects in in vivo studies [12,13]. The ginsenoside Rb3 alleviated the increase in malondialdehyde content and the decrease in superoxide dismutase and catalase activities an isoproterenol-induced animal model of myocardial injury [12]. The ginsenoside Rd increased the activity of glutathione peroxidase (GPx) and glutathione reductase (GR) in senescence-accelerated mice (SAM) [13]. However, there are virtually no reported studies that investigated the free radical scavenging activities of IH-901. To investigate the free radical scavenging activity of IH-901, we carried out assays that compared IH-901-induced changes in total antioxidant capacity, reductive potential, DPPH, nitric oxide (NO), superoxide, hydroxyl radical and ferric chloride-induced lipid peroxidation. All experimental parameters were compared to positive controls known antioxidants such as ascorbic acid and butylated hydroxyanisole (BHA).
IH-901 was obtained from the Central Research Institute, IL-HWA Co. Ltd. (Seoul, Korea). The purity of IH-901 was above 98%. Polyoxyethylenesorbitan monolaurate (Tween-20), 1,1-diphenyl-2-picryl-hydrazyl (DPPH), 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine), nicotinamide adenine dinucleotide (NADH), butylated hydroxyanisole (BHA), trichloracetic acid (TCA), phenazine methosulfate (PMS), nitroblue tetrazolium (NBT), ferric chloride (FeCl3), thiobarbituric acid (TBA), hydrogen peroxide (H2O2), linoleic acid, ferrous chloride (FeCl2), potassium ferricyanide [K3Fe(CN)6], 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), sodium nitroprusside and ammonium thiocyanate were purchased from Sigma-Aldrich (St. Louis, USA). All other chemicals were analytical grade and obtained from either Sigma or Merck (Korea Merck Limited, Seoul).
The antioxidant activity of IH-901 was determined according to the thiocyanate method [14]. IH-901, ascorbic acid and BHA in 2.5 mL of potassium phosphate buffer (0.04 M, pH 7.0) were added to a linoleic acid emulsion (2.5 mL) in potassium phosphate buffer. Five milliliters of the linoleic acid emulsion consisted of 17.5 µg Tween-20, 15.5 µL linoleic acid, and 0.04 M potassium phosphate buffer. Five milliliter of the control consisted of a 2.5 mL linoleic acid emulsion and 2.5 mL potassium phosphate buffer. The mixed solution was incubated at 37℃ in a glass flask and in the dark. After the mixture was stirred for 3 min, the peroxide value was determined by reading the absorbance at 500 nm in a PowerWave XS spectrophotometer (BioTek, Seattle, USA), after reaction with FeCl2 and thiocyanate at intervals during the incubation. During linoleic acid oxidation, peroxides formed. These compounds oxidize Fe2+ to Fe3+. The Fe3+ ions form complexes with SCN-, which has a maximum absorbance at 500 nm. Therefore, a high absorbance indicates high linoleic acid oxidation. Solutions without IH-901 or standards were used as controls. All data regarding total antioxidant activity are the average of duplicate analyses. The inhibition of lipid peroxidation as a percentage was calculated by the following equation:
Inhibition (%)=[(A0-A1)/A0]×100
where A0 was the absorbance of the control reaction and A1 was the absorbance in the presence of IH-901.
The reductive potential of IH-901 was determined according to a method that was previously described [15]. Different concentrations of IH-901 (1.56-50 µg/mL) in 1 mL of distilled water were mixed with phosphate buffer (2.5 mL, 0.2M pH 6.6) and potassium ferricyanide [K3Fe(CN)6, 2.5 mL, 1%]. The mixture was incubated at 50℃ for 20 min. An aliquot (2.5 mL) of TCA (10%) was added to the mixture, which was then centrifuged for 10 min at 1000×g (LX-131; TOMY Tech. Co., Seattle, USA). The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm in a PowerWave XS spectrophotometer (BioTek). An increase in absorbance of the reaction mixture indicated a greater reductive potential.
Measurement of superoxide anion scavenging activity of IH-901 was based on a previously described method [16]. Superoxide radical is generated in PMS-NADH systems by oxidation of NADH and assayed by the reduction of nitroblue tetrazolium (NBT). In this experiment, superoxide radicals were generated in 3 mL of Tris-HCl buffer (16 mM, pH 8.0) containing 1 mL of NBT (50 µM), 1mL NADH (78 µM) and a sample of IH-901 (from 6.25 to 100 µg/mL) in water. The reaction was started by adding 1 mL of PMS (10 µM) to the mixture. The reaction mixture was incubated at 25℃ for 5 min, and the absorbance at 560 nm in a PowerWave XS spectrophotometer was measured against blank samples. Ascorbic acid was used as a control. A decrease in absorbance of the reaction mixture indicated increased superoxide anion scavenging activity. The percentage inhibition of superoxide anion generation was calculated using the following formula:
Inhibition (%)=[(A0-A1)/A0]×100
where A0 was the absorbance of the control, and A1 was the absorbance with IH-901.
Hydroxyl radical scavenging activity was determined according to the deoxyribose method [17]. The scavenging activity of IH-901 was measured by the competition between deoxyribose and IH-901 for the hydroxyl radicals generated from a Fe3+/ascorbate/EDTA/H2O2 system. Briefly, for the hydroxyl radical system, the reaction mixture containing different concentrations of IH-901 (from 3.125 to 100 µg/mL), 2.8 mM deoxyribose, 0.1 mM FeCl3, 0.1 mM ascorbic acid, 0.1 mM EDTA and 1 mM H2O2 in phosphate buffer (20 mM, pH 7.4) were incubated in a water bath at 37℃ for 30 min. The extent of deoxyribose degradation was measured by the thiobarbituric acid (TBA) method. TBA (300 µL, 0.6%) and phosphoric acid (1 mL) were added to the mixture which was heated at 100℃ for 45 min and the absorbance at 520 nm in a PowerWave XS spectrophotometer was measured against blank samples. The hydroxyl radical scavenging activity was calculated using the following formula:
Inhibition (%)=[(A0-A1)/A0]×100
where A0 is the absorbance of the control, and A1 is the absorbance of the sample.
The free radical scavenging activity of IH-901 was measured by a DPPH scavenging assay using a previously described method [18]. Briefly, 0.1 mM solution of DPPH in ethanol was prepared. Then, 1 mL of this solution was added to 3 mL of IH-901 solutions at different doses (from 3.125 to 100 µg/mL). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance was measured at 517 nm in the PowerWave XS spectrophotometer. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The DPPH radical concentration was calculated using the following formula:
Inhibition (%)=[(A0-A1)/A0]×100
where A0 is the absorbance of the control, and A1 is the absorbance of the sample.
ABTS forms a relatively stable free radical, which decolorizes in its non-radical form [19]. Spectrophotometric analysis of ABTS scavenging activity was done according to a previously described method [20]. ABTS radical cations were produced by reacting 2 mM ABTS in distilled water with 70 mM potassium persulfate (K2S2O8), stored in the dark at room temperature for 24 h. Then, 1 mL of ABTS radical cation solution was added to 1 mL of IH-901 solution in DMSO at different concentrations (from 6.25 to 100 µg/mL). The absorbance was recorded 30 min after mixing and the percentage of radical scavenging was calculated for each concentration relative to a blank containing no scavenger. The extent of decolorization was calculated as a percentage reduction in absorbance. For preparation of a standard curve, different concentrations of ABTS cations were used. The ABTS concentration (mM) in the reaction medium was calculated from the following calibration curve, determined by linear regression (r2: 0.9999):
Absorbance (λ734 nm)=0.0004[ABTS+]+0.0391
The scavenging capability of test compounds was calculated using the following formula:
Inhibition (%)=[(A0-A1)/A0]×100
where A0 is the absorbance of the control, and A1 is the absorbance of the sample.
Nitric oxide scavenging activity was analyzed using a previously reported method [21]. We mixed 0.5 mL of 10 mM sodium nitroprusside in phosphate-buffered saline with 0.5 mL of different concentrations (from 6.25 to 100 µg/mL) of the IH-901 and incubated the mixture in the dark at room temperature for 150 min. Controls were run as above but the sample was replaced with an equal amount of water. After the incubation period, 1 mL of 0.33% sulfanilic acid reagent (in 20% glacial acetic acid) was added to 0.5 mL of the reaction mixture. After a 5-min incubation, 1 mL of 0.1% naphthylethylenediamine dihydrochloride was added, mixed and incubated for 30 min at 25℃. The absorbance of the chromophore formed was read at 540 nm. Ascorbic acid was used as a positive control and results are expressed as percentage inhibition of nitric oxide.
The scavenging capability of test compounds was calculated using the following formula:
Inhibition (%)=[(A0-A1)/A0]×100
where A0 is the absorbance of the control, and A1 is the absorbance of the sample.
Lipid peroxidation was measured by the method of Halliwell and Gutteridge [22]. Liver homogenates were prepared by grinding fresh normal liver from Sprague-Dawley rats in phosphate bufferd saline, pH 7.4 (10% weight/volume). The homogenate was centrifuged at 3,000 rpm for 15 min and the clear supernatant was taken for analysis. An aliquot (100 µL) of IH-901 solution at different concentrations was added to 0.5 mL of the rat liver homogenate. Then, peroxidation was initiated by adding 100 µL of 0.2 mM ferric chloride. The tubes were incubated at 37℃ for 30 min. The reaction was stopped by adding 2 mL of ice-cold hydrochloric acid (0.25 N) containing 15% trichloroacetic acid, 0.38% thiobarbituric acid, and 8.1% SDS. The reaction mixture was annealed at 95℃ for 30 min. The samples were cooled and centrifuged and the absorbance of the supernatants was measured at 532 nm. Inhibition of lipid peroxidation by the sample was calculated using the following formula:
Inhibition (%)=[(A0-A1)/A0]×100
where A0 is the absorbance of the control (distilled water), and A1 is the absorbance of the sample.
All data on total antioxidant activity are the average of duplicate analyses. The data were recorded as mean±SD and analyzed by SPSS (version 11.5 for Windows; SPSS Inc., Chicago, IL, USA). One-way analysis of variance was performed. Significant differences between means were determined by Dennett's multiple range test. P<0.05 was regarded as significant.
There are numerous methods for evaluation of antioxidant activity. The ones most commonly used include: total antioxidant activity, reductive potential, DPPH free radical scavenging assay, metal chelating, reactive oxygen species (such as hydrogen peroxide, superoxide, and hydroxyl radical) quenching assay [23]. Total antioxidant capacity of IH-901, BHA and ascorbic acid were determined by the thiocyanate method. IH901 had an effective antioxidant activity at all doses. The effects of various amounts of IH-901 (6.25, 25 and 100 µg/mL) on peroxidation of a linoleic acid emulsion are shown in Figure 1A. The antioxidant activity of IH-901 increased in a dose-dependent manner. IH-901 (25 and 100 µg/mL) showed comparable antioxidant activity to that of 50 µg of ascorbic acid. The percentage inhibition by 6.25, 25 and 100 µg/mL IH-901 of peroxidation in the linoleic acid emulsion was 60.37, 83.17 and 85.33%, respectively, and the percentage inhibition by 50 µg/mL of ascorbic acid and BHA was found to be 84.93 and 88.03%.
The reductive potential of a compound may serve as a significant indicator of its potential antioxidant activity. The presence of antioxidants causes the reduction of the Fe3+/ferricyanide complex to the ferrous form. The yellow color of the test solution changes into various shades of green and blue depending on the reductive potential of the antioxidant samples [15]. Therefore, Fe2+ can be monitored by the absorbance at 700 nm. To measure the reductive potential, we investigated the Fe3+-Fe2+ transformation in the presence of IH-901 using the method of Oyaizu [15]. Figure 1B shows the reductive potential of IH-901 compared to BHA and ascorbic acid as standards. Higher absorbance of the reaction mixture indicated greater reductive potential. The reductive potential of BHA, ascorbic acid and IH-901 were increased with increasing sample concentrations. IH-901 had an effective reductive potential although it was lower than for BHA and ascorbic acid. These results suggest that IH-901 and the other two standards have outstanding ability to donate electrons to reactive free radicals, converting them into more stable non-reactive species and terminating the free radical chain. The reductive potential of IH-901 was 2 to 3 fold lower than that of BHA and ascorbic acid.
Radical scavenging activities are very important due to the deleterious role of free radicals in biological systems. The effect of antioxidants on DPPH radicals is thought to be due to their hydrogen donating ability [24]. DPPH radical scavenging activity was determined by the decrease in absorbance at 517 nm in the presence of BHA, ascorbic acid and IH-901. It is visually noticeable as a change in coloration from purple to yellow. The scavenging of DPPH radicals by BHA, ascorbic acid and IH-901 occurred in a dose-dependent manner from 3.125 to 100 µg/mL (Figure 2A). Scavenging of DPPH radicals decreased in the order: ascorbic acid>BHA>IH-901; at a concentration of 100 µg/mL, the inhibition was 97.54, 90.98 and 86.61%, respectively. Similarly, it was reported that ginseng efficiently scavenged DPPH radicals [3]. These results indicate that IH-901 is a remarkable scavenger of DPPH free radicals. The free radical scavenging activity of these agents increased with increasing concentrations of the agents. These data clearly indicate that IH-901 is a powerful inhibitor of free radical production and a free radical scavenger.
The superoxide anion is a free radical created from the normal process of energy generation in mitochondria, and superoxide anions are a precursor to active free radicals that have the potential to react with biological macromolecules and thereby induce tissue damage [25]. In the PMS/NADH-NBT system, the superoxide anion derived from dissolved oxygen from the PMS/NADH coupling reaction reduces NBT. In this method, superoxide anion reduces the yellow dye (NBT2+) to produce the blue formazan which is measured spectrophotometrically at 560 nm. Antioxidants are able to inhibit NBT formation [26]. Figure 2B shows the superoxide radical scavenging capacity of IH-901 and ascorbic acid. Bothe IH-901 and ascorbic acid showed concentration-dependent scavenging activity of superoxide radicals. The percentage inhibition of superoxide generation by 100 µg/mL ascorbic acid was 90.46%, which was greater than that of the same concentration of IH-901 (71.49%). The superoxide radical scavenging activity of 50 µg/mL of IH-901 was nearly equal to that of 33.2 µg/mL of ascorbic acid. These results indicate that IH-901 has a remarkable effect on superoxide radical removal.
The hydroxyl radical is the most reactive free radical and can be formed from superoxide anion and hydrogen peroxide in the presence of metal ions, such as copper or iron. It can react with many other compounds such as protein, DNA, unsaturated fatty acids and almost every biological membrane [27]. Hydroxyl radical scavenging was investigated using the Fenton reaction. As shown in Figure 2C, IH-901 dose dependently scavenged hydroxyl radicals. Less than 100 µg/mL of IH-901 effectively inhibited the formation of hydroxyl radicals linearly up to a maximum inhibition of 68.61±2.76%. The hydroxyl radical scavenging activity of 50 µg/mL of BHA was nearly equal to that of 37.8 µg/mL of IH-901. These results indicate that IH-901 can effectively scavenge hydroxyl radicals.
Generation of the ABTS cation forms the basis of one of the spectrophotometric methods of measuring radical scavenging. The assay involves a decolorization technique in which the radical is generated directly in a stable form prior to reaction with putative antioxidants [24]. As seen in Figure 2D, IH-901 scavenged ABTS radicals in a concentration-dependent manner (6.25-100 µg/mL). There was a decrease in the concentration of ABTS+ due to the scavenging capacity of IH-901. At 100 mg/mL, the scavenging effects of ascorbic acid, BHA and IH-901 were 96.40, 95.95 and 64.59%, respectively. The scavenging activity of 50 µg/mL of IH-901 was nearly equal to that of 17.79 µg/mL of BHA and 20.02 µg/mL of ascorbic acid. These results indicate that IH-901 scavenges ABTS cations.
Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates NO, which interacts with oxygen to produce nitrite ions that can be estimated using the Griess method [28]. Nitric oxide is a very unstable species under aerobic conditions. It reacts with O2 to produce stable products nitrate and nitrite, through intermediates such as NO2, N2O4 and N3O4. Nitric oxide is a potent pleiotropic mediator of physiological processes such as smooth muscle relaxation, neuronal signaling, inhibition of platelet aggregation and regulation of cell mediated toxicity. It is a diffusible free radical that plays many roles as an effector molecule in diverse biological systems including neuronal messenger, vasodilatation, antimicrobial and antitumor activities. Studies in animal models have suggested a role for NO in the pathogenesis of inflammation and pain, and NOS inhibitors have been shown to have beneficial effects on some aspects of the inflammation and tissue changes seen in models of inflammatory bowel disease [19,29].
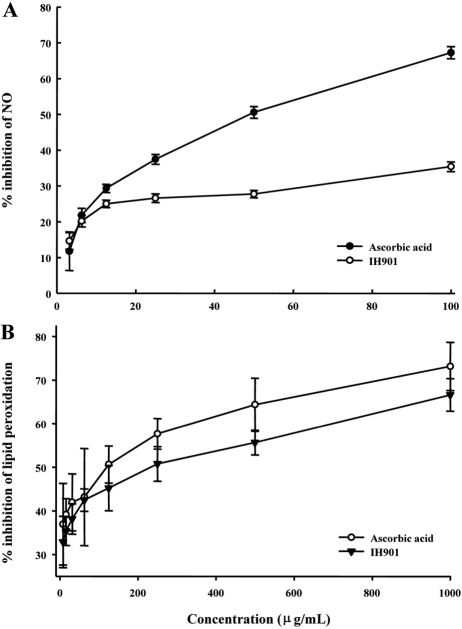
IH-901 showed mild nitric oxide scavenging compared to ascorbic acid (Figure 3A), Both IH-901 and ascorbic acid scavenged nitric oxide in a dose-dependent manner (3.125-100 µg/mL). Less than 12.5 µg/mL of IH-901 moderately inhibited the formation of nitric oxide linearly up to 25±1.01%; no further inhibition was observed at more than 12.5 µg/mL of IH-901. The nitric oxide scavenging activity of 12.5 µg/mL of IH-901 was nearly equal to that of 9.18 µg/mL of ascorbic acid. These results indicate that IH-901 has a modest effect on nitric oxide scavenging.
Rat liver lipids undergo rapid non-enzymatic peroxidation when incubated in the presence of FeCl3. Lipid peroxides are likely involved in numerous pathological events, including inflammation, metabolic disorders and cellular aging [14]. We evaluated the protective effect of IH-901 on normal rat liver treated with ferric chloride. The percentage inhibition of lipid peroxidation by 1 mg/mL IH-901 was 66.59%. The ratio at this concentration for ascorbic acid was 73.13% (Figure 3B). These results indicate that IH-901 inhibits lipid peroxidation. Since lipid peroxidation leads to cell death, the inhibition of lipid peroxidation has been considered as an index of antioxidant capacity [30].
IH-901 is a final intestinal bacterial metabolite of ginseng in humans. However, virtually no studies have investigated the antioxidant properties of IH-901. Therefore, the objectives of this study were to investigate total antioxidant activity, reductive potential, DPPH free radical scavenging, superoxide anion radical scavenging, hydroxyl radical scavenging, nitric oxide scavenging, ferric chloride-induced lipid peroxidation decreasing, and ABTS radical scavenging activities of IH-901. In vitro antioxidative effects of IH-901 were compared with the same dose of standard antioxidants such as BHA and ascorbic acid, which are commonly used by the pharmaceutical industry. The results of this study clearly indicate that IH-901 has powerful antioxidant effects in various antioxidant systems in vitro and that these effects were concentration dependent. The antioxidant mechanisms of IH-901 may be strong hydrogen donating ability, and its effectiveness as a scavenger of superoxide, hydroxyl radical, DPPH free radical, nitric oxide and ferric chloride-induced lipid peroxidations. However, the components responsible for the antioxidative activities of IH-901 are currently unclear. IH-901 could, if optimized, be used as a source of antioxidants for use in foods to replace synthetic antioxidants.
References
1. Aruoma O. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998; 75(2):199–212.

2. Cerutti PA. Oxidant stress and carcinogenesis. Eur J Clin Invest. 1991; 21(1):1–5. PMID: 1907547.

3. Jung CH, Seog HM, Choi IW, Park MW, Cho HY. Antioxidant properties of various solvent extracts from wild ginseng leaves. Food Sci Technol-LEB. 2006; 39(3):266–274.

4. Halliwell B. Antioxidant characterization : Methodology and mechanism. Biochem Pharmacol. 1995; 49(10):1341–1348. PMID: 7763275.
5. Jung CH, Seog HM, Choi IW, Cho HY. Antioxidant activities of cultivated and wild Korean ginseng leaves. Food Chem. 2005; 92(3):535–540.

6. Kang KS, Yamabe N, Kim HY, Okamoto T, Sei Y, Yokozawa T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol. 2007; 113(2):225–232. PMID: 17618072.

7. Hasegawa H, Sung JH, Huh JH. Ginseng intestinal bacterial metabolite IH901 as a new anti-metastatic agent. Arch Pharm Res. 1997; 20(6):539–544. PMID: 18982256.

8. Lee BH, Lee SJ, Hui JH, Lee S, Sung JH, Huh JD, Moon CK. In vitro antigenotoxic activity of novel ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1998; 64(6):500–503. PMID: 9741293.
9. Sung JH, Hasegawa H, Matsumiya S, Uchiyama M, Ha JY, Lee MS, Huh JD. Metabolism of ginseng saponins by human intestinal bacteria. Korean J Pharmacogn. 1995; 26:360–367.
10. Zhang D, Yasuda T, Yu Y, Zheng P, Kawabata T, Ma Y, Okada S. Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996; 20(1):145–150. PMID: 8903691.

11. Kang J, Lee Y, No K, Jung E, Sung J, Kim Y, Nam S. Ginseng intestinal metabolite-I (GIM-I) reduces doxorubicin toxicity in the mouse testis. Reprod Toxicol. 2002; 16(3):291–298. PMID: 12128103.

12. Wang T, Yu X, Qu S, Xu H, Han B, Sui D. Effect of ginsenoside Rb3 on myocardial injury and heart function impairment induced by isoproterenol in rats. Eur J Pharmacol. 2010; 636:121–125. PMID: 20371232.

13. Yokozawa T, Satoh A, Cho EJ. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J Pharm Pharmacol. 2004; 56(1):107–113. PMID: 14980007.

14. Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. Food Sci Technol-LEB. 2008; 41(6):1060–1066.

15. Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986; 44:307–315.
16. Liu F, Ooi VEC, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997; 60(10):763–771. PMID: 9064481.

17. Aruoma OI. Deoxyribose assay for detecting hydroxyl radicals. Methods Enzymol. 1994; 233:57–66.
18. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992; 40(6):945–948.

19. Shirwaikar A, Shirwaikar A, Rajendran K, Punitha IS. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull. 2006; 29(9):1906–1910. PMID: 16946507.
20. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10):1231–1237. PMID: 10381194.

21. Rai S, Wahile A, Mukherjee K, Saha BP, Mukherjee PK. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol. 2006; 104(3):322–327. PMID: 16239089.
22. Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 1986. 2nd ed. Tokyo: Japan Scientific Societies Press;p. 42–53.
23. Gülçin I, Küfrevioglu ÖI, Oktay M, Büyüukokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004; 90(2-3):205–215. PMID: 15013182.
24. Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology. 2006; 217(2-3):213–220. PMID: 16243424.

25. Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984; 219(1):1–14. PMID: 6326753.

26. Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. J Agric Food Chem. 2002; 50(23):6882–6890. PMID: 12405792.

27. Feng T, Du Y, Li J, Hu Y, Kennedy JF. Enhancement of antioxidant activity of chitosan by irradiation. Carbohydr Polym. 2008; 73(1):126–132.

28. Razali N, Razab R, Junit SM, Aziz AA. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem. 2008; 111(1):38–44.
29. Govindarajan R, Rastogi S, Vijayakumar M, Shirwaikar A, Rawat AK, Mehrotra S, Pushpangadan P. Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull. 2003; 26(10):1424–1427. PMID: 14519948.

30. Kim KT, Yoo KM, Lee JW, Eom SH, Hwang IK, Lee CY. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J Ethnopharmacol. 2007; 111(3):443–450. PMID: 17276636.
Figure 1
Total antioxidant capacity and reductive potential of IH-901, BHA and ascorbic acid. For total antioxidant capacity (A), briefly, IH-901 or standard samples in 2.5 mL of potassium phosphate buffer were added to a linoleic acid emulsion in potassium phosphate buffer. The mixed solution was incubated at 37℃ in a glass flask in the dark, and the peroxide value was determined by reading the absorbance at 500 nm after reaction with FeCl2 and thiocyanate at intervals during the incubation. For reductive potential (B), briefly, the different concentrations of IH-901 (1.56-50 µg/mL) in 1 mL of distilled water were mixed with phosphate buffer and potassium ferricyanide. An aliquot (2.5 mL) of TCA was added to the mixture, which was then centrifuged for 10 min at 1000×g. The upper layer of the solution was mixed with distilled water and FeCl3, and the absorbance was measured at 700 nm. BHA: butylated hydroxyanisole, IH-901: ginseng intestinal metabolite-I.
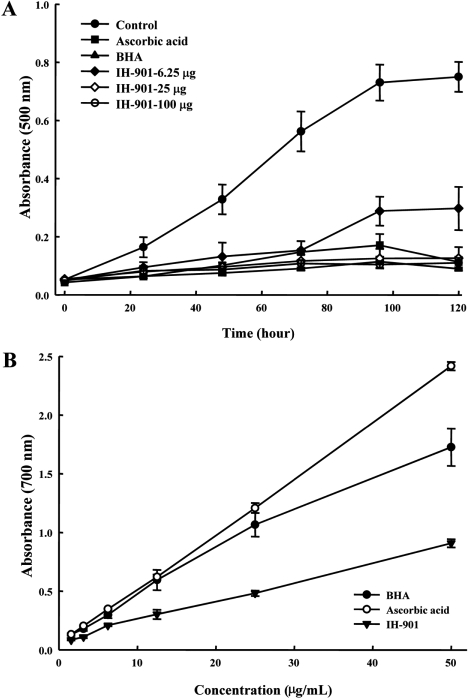
Figure 2
DPPH (A), superoxide (B), hydroxyl (C) and ABTS (D) radical scavenging activities of BHA, ascorbic acid and IH-901. The radical scavenging ability of varying concentrations (3.125-100 µg/mL) of BHA, ascorbic acid and IH-901 was analyzed by measuring their inhibitory effects on the absorbance of DPPH, superoxide, hydroxyl and ABTS radicals. The results are expressed as mean±SD of the % inhibition of the absorbance of DPPH, superoxide, hydroxyl and ABTS radicals. DPPH: 1,1-diphenyl-2-picrylhydrazyl, ABTS: 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), BHA: butylated hydroxyanisole, IH-901: ginseng intestinal metabolite-I.
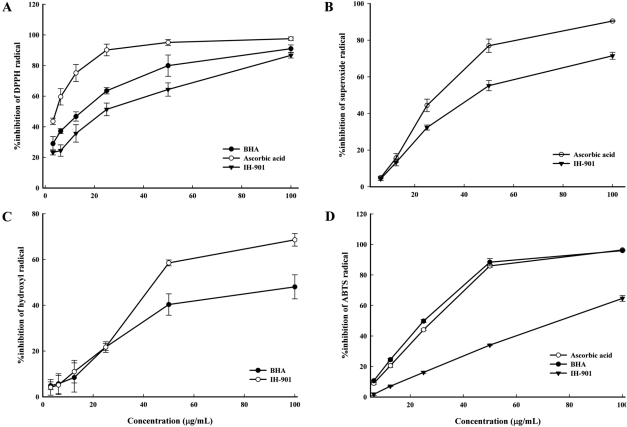
Figure 3
Percentage inhibition of nitric oxide radicals (A) and lipid peroxidation (B) in the presence of different concentrations (3.125-1000 µg/mL) of ascorbic acid and IH-901. Nitric oxide (NO) radical scavenging activity was analyzed by measuring their inhibitory effects on the absorbance of the NO reaction product. It was analyzed by measuring inhibition of the absorbance of the thiobarbituric acid reaction product. Lipid peroxide production measured by UV detection of pink chromogens of malondialdehyde (MDA) in respective control reactions were 3.17±0.77. The results are expressed as mean±SD of the % inhibition of the absorbance of the NO and MDA. IH-901: ginseng intestinal metabolite-I.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download