Abstract
The hexane extract of Rheum undulatum L. (HERL) has been shown to have anti-cancer activity in several cancers in vivo and in vitro. However, the anti-cancer activity of HERL and its molecular mechanism in human oral cancer cells has not been explored. Thus, the aim of this study was to elucidate the growth-inhibitory and apoptosis-inducing effects of HERL in HN22 and SCC15 oral cancer cell lines. This study shows that HERL inhibits oral cancer growth, decreases cell viability, and causes apoptotic cell death in HN22 and SCC15 cells, as characterized by morphological changes, nuclear condensation and fragmentation, the cleavage of PARP and the accumulation of cells in the sub-G1 phase. The treatment of oral cancer cells with HERL also resulted in decreased expression of specificity protein (Sp1) and its downstream protein, survivin. Therefore, our results suggest that the regulation of Sp1 and survivin plays a critical role in HERL-induced apoptosis in human oral cancer cells.
Oral and pharyngeal cancer is the sixth most common cancer world-wide and two-thirds of these cases occur in developing countries (Parkin et al, 2005). Despite advances in cancer diagnosis and treatment, there has been little improvement in the 5-year survival rate of oral cancer patients in the last few decades. Because of this, various approaches have been used in the clinical treatment of oral cancer patients in the last three decades, from non-targeted chemotherapy to highly targeted pharmacological inhibitors and specific monoclonal antibodies (Hamakawa et al, 2008; Scully and Bagan, 2009). Although targeted therapies yield better outcomes than non-targeted therapies, frequent treatment failure suggests the need for new strategies for this disease, including new drugs or targets.
Rheum undulatum L. (RL), a well-known traditional Chinese medicine, has been widely used for thousands of years in China for the treatment of many diseases (He et al, 2009). In particular, the root of the species is widely used as a purgative and anti-inflammatory agent in East Asia (Chang et al, 1996; Kuo et al, 2001; Yu et al, 2008). It has been also reported that RL is used traditionally in Korea for the treatment of dental disease (Kim et al, 2010). Recently, it was found that RL contains many anthraquinones and their glycosides, including aloe-emodin, rhein, emodin, and chrysophanol, which are thought to be the major active components for inhibiting tumor growth in various cancer cells (Cha et al, 2005; Huang et al, 2006; Huang et al, 2007; Dorsey and Kao, 2007). However, there has been no study performed to investigate the effect of RL on the growth of human oral cancer cells.
In the present study, we examined the effect of the hexane extract of Rheum undulatum L. (HERL) on the growth of oral cancer cells and the molecular targets for HERL-induced apoptosis. The results demonstrated that HERL induced apoptotic cell death to inhibit the proliferation of oral cancer cells and the down-regulation of Sp1 and survivin mediated the anti-cancer activity of HERL.
The antibodies for poly(ADP-ribose) polymerase (PARP) and survivin were obtained from BD Biosciences (San Jose, CA, USA) and Cell Signaling Technology (Danvers, MA, USA), respectively. Antibodies for Sp1 and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). 4'-6-Diamidino-2-phenylindole (DAPI) and propidium iodide (PI) were acquired from Sigma-Aldrich (St. Louis, MO, USA). HERL was kindly supplied by professor Jeon JG (Jeonbuk National University, Jeonju, Korea).
The HN22 and SCC15 human oral cancer cell lines were obtained from the School of Dentistry, Dankook University (Cheonan, Korea). The cells were cultured in Dulbecco's modified essential medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 units/mL penicillin and 100 µg/mL streptomycin (WelGENE, Daegu, Korea) in a humid atmosphere of 5% CO2. Equal numbers of cells were seeded and allowed to attach overnight. The cells were treated with 0.1% NaCl or HERL (20, 40 or 60 µg/mL) diluted in DMEM with 5% FBS for 48 h.
The effects of HERL on cell viability were estimated using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. The cells were seeded in 96-well plates and incubated with various concentrations of HERL. After the treatment with HERL for 48 h, 30 µL of 3-(4,5-dimethylthiazol-20yl)-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium) (MTS) solution was added to each well and the cells were incubated for 2 h at 37℃. MTS solution was analyzed using a microplate reader (BioTeck Instruments, Winooski, VT, USA) at 490 and 690 nm (background).
After treatment with HERL for 48 h, the detached HN22 and SCC15 cells were collected and combined with the adherent cells that had been released by trypsinization. The cells were fixed in 70% ethanol overnight at -20℃. The cells were subsequently stained with 0.02 mg/mL PI. The data were acquired using a FACScan flow cytometer.
The number of cells with nuclear condensation and fragmentation was measured using DAPI staining. After treatment with HERL or 0.1% NaCl (control), the HN22 and SCC15 cells were harvested by trypsinization. The cells were resuspended in PBS, deposited on poly-L-lysine-coated slides, stained with DAPI solution (2 µg/mL) and observed under a fluorescence microscope.
After treatment with HERL, HN22 and SCC15 cells were harvested. The protein supernatant fractions were subjected to SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes and blocked with 5% skim milk followed by hybridization with the indicated antibodies. After incubation with the horseradish peroxidase (HRP)-conjugated secondary antibody, the immunoreactive protein bands were observed using a chemiluminescence detection kit.
We first investigated the effect of HERL on the growth of HN22 and SCC15 cells. HERL-treated cells were detached from the dishes compared to 0.1% NaCl-treated cells and these morphological changes occurred in a dose-dependent manner (Figure 1A). We also measured the effect of HERL on cell viability in the MTS assay. The results showed dose-dependent decreases of cell viability in HN22 and SCC15 cells (Figure 1B). These results suggest that HERL has a growth-inhibitory effect on HN22 and SCC15 cells.
To examine whether HERL induced apoptosis, the number of cells accumulated in the sub-G1 phase of the cell cycle was counted by flow cytometric analysis. As shown in Figure 2, the sub-G1 population of HERL-treated cells accumulated in a dose-dependent manner compared to the vehicle control. To confirm the induction of apoptosis by HERL in HN22 and SCC15 cells, DAPI staining was performed and showed the fragmentation and condensation of nucleus in the cells treated with HERL (20, 40 and 60 µg/mL) for 48 h compared to the control (Figure 3). We next determined the level of PARP cleavage by Western blot analysis (Figure 4). After HERL treatment for 48 h, cleaved PARP was detected in the HN22 and SCC15 cells. Thus, we clearly showed that HERL induced apoptosis in HN22 and SCC15 cells to inhibit cell growth.
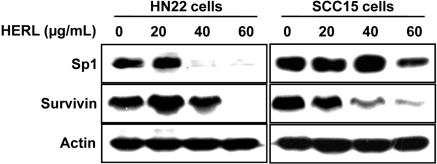
In previous studies, it was reported that targeting of Sp1 protein is a good treatment strategy for various cancer cells (Lou et al, 2005; Safe and Abdelrahim, 2005; Abdelrahim et al, 2006; Papineni et al, 2009; Lu and Archer, 2010) and Sp1 protein regulates survivin protein as the downstream protein to induce apoptosis in cancer cells (Shin et al, 2010). Therefore, we assessed whether HERL affects Sp1 and survivin protein to induce apoptotic cell death in HN22 and SCC15 cells. As shown in Figure 5, HERL-treated HN22 and SCC15 cells exhibited decreased Sp1 and survivin expression in a dose-dependent manner. These results suggest that HERL might inhibit Sp1 and survivin protein to exert its apoptotic activity in HN22 and SCC15 cells.
Natural extracts from edible plants have been identified as excellent candidates for cancer therapeutics based on safety and efficacy (Stewart et al, 2003). Recently, Rheum undulatum L. was screened for anti-cancer activity in vitro using human breast, ovary, cervix and lung cancer cell lines (Kang et al, 2008). In that study, emodin showed strong cytotoxicity in both estrogen receptor (ER)-positive and -negative breast cancer cell lines (Kang et al, 2008). In addition, HERL was shown to inhibit angiogenic activity in a zebrafish model (He et al, 2009). These studies suggest that HERL has potential benefits for the treatment of various cancers. However, the anticancer activity of HERL against oral cancer cells has not been well established. Therefore, we focused on the anticancer activity of HERL in human oral cancer cells. Our first purpose was to examine the anti-proliferative and pro-apoptotic effects of HERL on oral cancer cell lines and the other was to determine its molecular mechanism in HERL-induced apoptosis. First, we found that HERL decreased the number of viable cells in a dose-dependent manner, showing that HERL has an inhibitory effect on cell growth in HN22 and SCC15 cells. We found that HERL induced the apoptosis of HN22 and SCC15 cells as evidenced by the accumulation of sub-G1 phase cells, distinct chromatin condensation and nuclear fragmentation of the nucleus and the cleavage of PARP protein, a typical apoptotic marker.
Sp1 is a member of the mammalian transcription factor family that binds to GC-rich sites containing GC-boxes. Recently, it was reported that Sp1 protein is over-expressed in many human tumors and cancer cell lines (Zannetti et al, 2000; Chiefari et al, 2002; Wang et al, 2003; Hosoi et al, 2004; Yao et al, 2004). Sp1 has also been implicated in multiple cell processes, including apoptosis, through activation of the FAS-ligand (Kavurma et al, 2001; Sun et al, 2001). In addition, survivin, an inhibitor of apoptosis and a key regulator of mitosis, is up-regulated in a variety of cancer cells and is often associated with a worse prognosis (Sun et al, 2001). Interestingly, several studies have shown that survivin expression can be regulated by Sp1 protein because it contains highly GC-rich sequences within its promoters (Li and Altieri, 1999; Wu et al, 2005; Li et al, 2006; Shim et al, 2010). Therefore, we examined the effects of HERL on the expression of Sp1 and its downstream target, survivin, as the key molecular factors in human oral cancer cells. Our results show that HERL decreases the expression levels of Sp1 and survivin proteins, suggesting that HERL inhibits the growth and induces apoptosis in HN22 and SCC15 cells through the regulation of Sp1 and survivin.
In summary, our data showed HERL exerts pro-apoptotic and anti-proliferative effects in HN22 and SCC15 human oral cancer cells and that Sp1 and survivin play important signaling roles in HERL-induced apoptosis. Therefore, we suggest that HERL is a promising anti-cancer drug candidate for the effective treatment of oral cancer.
References
1. Abdelrahim M, Baker CM, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006; 98:855–868. PMID: 16788159.

2. Cha TL, Qiu L, Chen CT, Wen Y, Hung MC. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005; 65:2287–2295. PMID: 15781642.

3. Chang CH, Lin CC, Yang JJ, Namba T, Hattori M. Anti-inflammatory effects of emodin from ventilago leiocarpa. Am J Chin Med. 1996; 24:139–142. PMID: 8874670.

4. Chiefari E, Brunetti A, Arturi F, Bidart JM, Russo D, Schlumberger M, Filetti S. Increased expression of AP2 and Sp1 transcription factors in human thyroid tumors: a role in NIS expression regulation? BMC Cancer. 2002; 2:35. PMID: 12475396.

5. Dorsey JF, Kao GD. Aloe(-emodin) for cancer? More than just a comforting salve. Cancer Biol Ther. 2007; 6:89–90. PMID: 17297301.

6. Hamakawa H, Nakashiro K, Sumida T, Shintani S, Myers JN, Takes RP, Rinaldo A, Ferlito A. Basic evidence of molecular targeted therapy for oral cancer and salivary gland cancer. Head Neck. 2008; 30:800–809. PMID: 18429007.

7. He ZH, He MF, Ma SC, But PP. Anti-angiogenic effects of rhubarb and its anthraquinone derivatives. J Ethnopharmacol. 2009; 121:313–317. PMID: 19061946.

8. Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A, Nagawa H, Suzuki N. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol. 2004; 25:461–468. PMID: 15254745.

9. Huang Q, Shen HM, Shui G, Wenk MR, Ong CN. Emodin inhibits tumor cell adhesion through disruption of the membrane lipid Raft-associated integrin signaling pathway. Cancer Res. 2006; 66:5807–5815. PMID: 16740720.

10. Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007; 27:609–630. PMID: 17022020.

11. Kang SC, Lee CM, Choung ES, Bak JP, Bae JJ, Yoo HS, Kwak JH, Zee OP. Anti-proliferative effects of estrogen receptor-modulating compounds isolated from Rheum palmatum. Arch Pharm Res. 2008; 31:722–726. PMID: 18563353.

12. Kavurma MM, Santiago FS, Bonfoco E, Khachigian LM. Sp1 phosphorylation regulates apoptosis via extracellular FasL-Fas engagement. J Biol Chem. 2001; 276(7):4964–4971. PMID: 11053446.

13. Kim JE, Kim HJ, Pandit S, Chang KW, Jeon JG. Inhibitory effect of a bioactivity-guided fraction from Rheum undulatum on the acid production of Streptococcus mutans biofilms at sub-MIC levels. Fitoterapia. 2010; e-published.

14. Kuo YC, Meng HC, Tsai WJ. Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm Res. 2001; 50:73–82. PMID: 11289657.

15. Li F, Altieri DC. Transcriptional analysis of human survivin gene expression. Biochem J. 1999; 344(Pt 2):305–311. PMID: 10567210.

16. Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y, Yan B. The expression of antiapoptotic protein survivin is transcriptionally upregulated by DEC1 primarily through multiple sp1 binding sites in the proximal promoter. Oncogene. 2006; 25:3296–3306. PMID: 16462771.

17. Lou Z, O'Reilly S, Liang H, Maher VM, Sleight SD, McCormick JJ. Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res. 2005; 65(3):1007–1017. PMID: 15705902.
18. Lu S, Archer MC. Sp1 coordinately regulates de novo lipogenesis and proliferation in cancer cells. Int J Cancer. 2010; 126(2):416–425. PMID: 19621387.
19. Papineni S, Chintharlapalli S, Abdelrahim M, Lee SO, Burghardt R, Abudayyeh A, Baker C, Herrera L, Safe S. Tolfenamic acid inhibits esophageal cancer through repression of specificity proteins and c-Met. Carcinogenesis. 2009; 30(7):1193–1201. PMID: 19406933.

20. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108. PMID: 15761078.

21. Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005; 41(16):2438–2448. PMID: 16209919.

22. Scully C, Bagan JV. Recent advances in oral oncology 2008; squamous cell carcinoma imaging, treatment, prognostication and treatment outcomes. Oral Oncol. 2009; 45:e25–e30. PMID: 19249236.

23. Shim JH, Shin JA, Jung JY, Choi KH, Choi ES, Cho NP, Kong G, Ryu MH, Chae JI, Cho SD. Chemopreventive effect of tolfenamic aicd on KB human cervical cancer cells and tumor xenograft by down regulating specificity protein 1. Eur J Cancer Prev. 2010; e-published.
24. Shin JA, Shim JH, Jeon JG, Choi KH, Choi ES, Cho NP, Cho SD. Apoptotic effect of Polygonum Cuspidatum in oral cancer cells through the regulation of specificity protein 1. Oral Dis. 2010; e-published.

25. Stewart JR, Artime MC, O'Brian CA. Resveratrol: a candidate nutritional substance for prostate cancer prevention. J Nutr. 2003; 133(7):2440S–2443S. PMID: 12840221.

26. Sun Y, Giacalone NJ, Lu B. Terameprocol (Tetra-O-Methyl Nordihydroguaiaretic Acid), an Inhibitor of Sp1-Mediated Survivin Transcription, Induces Radiosensitization in Non-small Cell Lung Carcinoma. J Thorac Oncol. 2011; 6(1):8–14. PMID: 21107289.

27. Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003; 9(17):6371–6380. PMID: 14695137.
28. Wu J, Ling X, Pan D, Apontes P, Song L, Liang P, Altieri DC, Beerman T, Li F. Molecular mechanism of inhibition of survivin transcription by the GC-rich sequence-selective DNA binding antitumor agent, hedamycin: evidence of survivin down-regulation associated with drug sensitivity. J Biol Chem. 2005; 280:9745–9751. PMID: 15637054.
29. Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004; 10:4109–4117. PMID: 15217947.

30. Yu HM, Liu YF, Cheng YF, Hu LK, Hou M. Effects of rhubarb extract on radiation induced lung toxicity via decreasing transforming growth factor-beta-1 and interleukin-6 in lung cancer patients treated with radiotherapy. Lung Cancer. 2008; 59:219–226. PMID: 17870203.

31. Zannetti A, Del Vecchio S, Carriero MV, Fonti R, Franco P, Botti G, D'Aiuto G, Stoppelli MP, Salvatore M. Coordinate up-regulation of Sp1 DNA-binding activity and urokinase receptor expression in breast carcinoma. Cancer Res. 2000; 60:1546–1551. PMID: 10749121.
Figure 1
Morphological changes and the viability of HN22 and SCC15 cells after treatment with hexane extract of Rheum undulatum L. (HERL). A, Photomicrographs of HN22 and SCC15 cells treated with 20, 40 and 60 µg/mL of HERL for 48 h. B, Cell viability was determined using MTS assay. The graph was representative of three independent experiments and bar is mean±SD. *P<0.05 compared to control group.
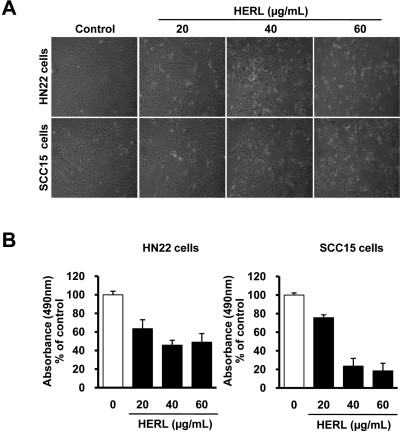
Figure 2
The effect of hexane extract of Rheum undulatum L. (HERL) on cell accumulation in sub-G1 phase. The sub-G1 population was measured by PI staining and flow cytometry analysis. The bar graph represents three independent experiments and mean±SD. *P<0.05 compared to control group.
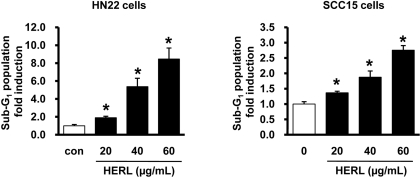
Figure 3
The effect of hexane extract of Rheum undulatum L. (HERL) on the condensation and fragmentation of nucleus in HN22 and SCC15 cells. Fluorescence microscopy images of DAPI-stained HN22 and SCC15 cells showing the appearance of apoptotic morphology.
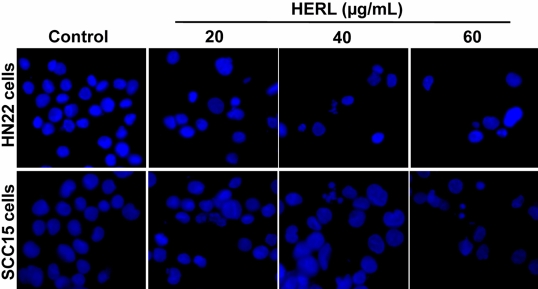
Figure 4
The effect of hexane extract of Rheum undulatum L. (HERL) on the cleavage of poly(ADP-ribose) polymerase (PARP) in HN22 and SCC15 cells. HN22 and SCC15 cells were treated with 20, 40 or 60 µg/mL of HERL for 48 h, and then the protein levels of cleaved PARP were determined by Western blot analysis. Actin was used to normalize the protein loading from each treatment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download