Abstract
Diabetic neuropathy is one of the most frequent and troublesome complications of diabetes. Although there has been a continuous increase in the incidence of diabetic neuropathy, treatments have yet to be found that effectively treat diabetic neuropathy. Neurotrophic factors are proteins that promote the survival of specific neuronal populations. They also play key roles in the regeneration of peripheral nervous system. Recent evidence from diabetic animal models and human diabetic subjects suggest that reduced availability of neurotrophic factors may contribute to the pathogenesis of diabetic neuropathy. One way to reverse this effect is to take advantage of the finding that bone marrow derived mesenchymal stem cells (BM-MSCs) promote peripheral nerve repair and the functioning of neurotrophic factors. Therefore, we speculated that treatment with BM-MSCs could be a viable therapeutic strategy for diabetic neuropathy. The present study was designed to examine the possible beneficial effect of BM-MSCs on functions of neurotrophic factors in diabetic neuropathy. To assess this possibility, we used an in vivo streptozotocin-induced diabetic neuropathy mouse model. Quantitative real-time polymerase-chain reacion showed that BM-MSCs significantly increase expression levels of neurotrophic factors. Also, BM-MSCs ameliorated nerve conduction velocity in streptozotocin-treated mice. These results may help to elucidate the mechanism by which BM-MSCs function as a cell therapy agent in diabetic neuropathy.
Diabetic neuropathy (DN) is the most common complication in diabetic patients. DN may not only cause various problems in daily life, but also, it may affect their prognosis [1,2]. Diabetic patients suffer from various symptoms of DN such as spontaneous pain, hyperalgesia and diminished sensation. DN is the major reason for loss of protective limb mechanical sensation, traumatic ulceration injures and, therefore, amputations [3]. A number of mechanisms have been proposed to link chronic hyperglycemia to diabetes-induced deficits in motor and sensory nerve conduction velocities and small fiber sensory neuropathy [4]. Although there has been a continuous increase in the incidence of DN, current treatments have yet to effectively treat DN.
The neurotrophin family of neurotrophic factors (NTFs) is a family of structurally and functionally related peptides that mediate potent survival and differentiation effects on a wide variety of neuronal populations in the nervous systems [5]. But only a few members of the family have particular importance with regard to the peripheral nervous system (PNS). Members of this family include brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and neurotrophin-3 (NT-3). These factors may stimulate nerve regeneration or collateral sprouting, as well as enhance the normal physiological functions of surviving neurons. NTFs including NGF and NT-3 are trophic for specific neuronal populations in the PNS. This specificity is determined by the receptors expressed by each neuronal population [6]. NTFs function by binding to 2 classes of receptors: tyrosine kinase receptors (Trk) and p75 receptors [7]. The p75 receptor binds all NTFs with similar affinity, but with different kinetics [8]. In contrast, the Trk receptors are more specific. Trk A is expressed on small-fiber sensory and sympathetic neurons and mediates most of the biological effects of NGF [9]. Trk C is the receptor for NT-3 [10]. Also, previous studies have demonstrated that neurotrophins are key mediators of the myelination program in the PNS [11-13]. Disturbed axonal growth in diabetes has been ascribed in part to the reduction of NTFs such as NGF and NT-3 in peripheral nerves and circumambient muscles and to decreased expression of their receptors in dorsal root ganglion cells [14-16].
Diabetes induced neurotrophic dysfunction may contribute to the pathogenesis of DN. We speculated that bone marrow-derived mesenchymal stem cells (BM-MSCs) could be a viable therapeutic strategy for DN because BM-MSCs have been reported to promote axonal regeneration and functional recovery of NTFs in peripheral nerve repair [17-19]. Also, transplantation of BM-MSCs might modulate neurotrophic functions in streptozotocin (STZ)-treated DN mice. Therefore, we injected BM-MSCs into the hind limb muscles of DN mice. Interestingly, this therapy increased expression levels of NTFs and ameliorated nerve conduction deficits in DN mice.
Six-week-old male BALB/c mice were allowed to adapt to the experimental animal facility for 2 weeks. At 8 weeks of age they were treated with STZ (Sigma-Aldrich, St. Louis, MO, USA) to induce diabetes. After anesthesia with a combination of 100 mg/kg ketamine and 10 mg/kg xylazine, mice were injected with 60 mg/kg STZ in 50 mM sodium citrate buffer, pH 4.5, daily for 5 days [20]. Control mice were treated with daily injections of citrate buffer. One week after the last injection, using a glucometer, serum glucose was measured in blood samples taken from mouse tail veins. Mice with plasma glucose concentrations of at least 250 mg/dL were selected as the STZ-induced diabetes group. Mice were housed in a room maintained under controlled temperature and humidity on a 12 hour/12 hour light/dark cycle. All procedures were done in accordance with an animal protocol approved by the Kyungpook National University Institutional Animal Care and Use Committee (IACUC).
Tibias and femurs were dissected from 4- to 6-week-old BALB/c mice. Bone marrow was harvested, and single-cell suspensions were obtained using a 40-µm cell strainer (Becton-Dickinson and Company, Franklin Lakes, NJ, USA). Approximately 106 cells were plated in 25-cm2 flasks containing (i) MesenCult™ MSC Basal Medium, (ii) Mesenchymal Stem Cell Stimulatory Supplements (Stem Cell Technologies, Vancouver, BC, Canada) and (iii) antibiotics as in our previous report [21]. The cell cultures were grown for 1 week, and the population that was adherent to plastic (BM-MSCs) was used for subsequent experiments.
Twenty weeks after the induction of diabetes, we injected BM-MSCs [1×106 cells/100 µL phosphate-buffer saline (PBS) per limb] or the same volume of PBS into the hind limb muscle percutaneously along the course of the sciatic nerve at 4 sites using a 30-gauge needle [22]. Two and 4 weeks later, the following parameters were measured.
Mice were anesthetized with a combination of 100 mg/kg ketamine and 10 mg/kg xylazine to prevent discomfort. Body temperature was maintained at 37℃ using a warming pad to ease animal stress from the anesthetic. The electrodes were cleaned with 70% alcohol between animals to maintain a pathogen-free status. The sciatic-tibial motor nerve conduction velocity (MNCV) between the ankle and the sciatic notch was determined with a recorder and bioamp (AD Instruments, ML820 and ML132, Castle Hill, Australia). Briefly, sciatic nerve was stimulated proximally at the sciatic notch and distally at the ankle via electrodes previously inserted through the skin with supramaximal stimulation (25 mA, 0.02 msec duration, AD Instruments, ML155). MNCV was calculated by dividing the distance between stimulating electrodes by the average latency difference between the initial onset to maximum negative peaks of the compound muscle action potentials evoked from two sites.
Tissue RNA samples were extracted from sciatic nerve and femoral muscles of diabetic mice 2 and 4 weeks after transplantation of STZ using RNeasy Lipid Tissue Mini kits (Qiagen Korea, Seoul, Korea). The RNA concentration was determined using a Nanodrop ND-1000 spectrophotometer. Five µg of each RNA was converted to cDNA using the sprint RT complete-oligo (dT) (Clontech, Palo Alto, CA, USA) according to the manufacturer's guide. The cDNA was quantified using the QuantiTect SYBR Green PCR Kit (Qiagen Korea). For each investigated transcript, a mixture of the following reaction components was prepared at the indicated end-concentrations: forward primer (10 pM), reverse primer (10 pM) and QuantiTect SYBR Green PCR Master mix. The 15 µL master-mix was added to a 0.1 mL tube and 5 µL, containing 100 ng reverse transcribed total RNA, was added as a PCR template. The tubes were closed, centrifuged and placed into a Corbett research RG-6000 real-time PCR machine (Corbett Life Science, Sydney, Australia). The following primers were used: NGF (forward: 5'-TCAGTGTG TGGGTTGGAGAT-3', reverse: 5'-CCACTCTCAACAGGATT GGA-3'), NT-3 (forward: 5'-TTCTGCCACGATCTTACAGG-3', reverse: 5'-GGCAAACTCCTTTGATCCAT-3').
To determine the impact of transplantation of BM-MSCs on the sciatic nerve, we measured sciatic MNCV 12, 14 and 16 weeks after STZ treatment. At baseline, 12 weeks after induction of diabetes, sciatic MNCV was significantly delayed in STZ-treated mice compared with normal mice, indicating development of diabetic neuropathy (Figure 1A). STZ-treated mice were randomly assigned to BM-MSCs or PBS injection groups and were injected intramuscularly around the sciatic nerves. A significant improvement in sciatic MNCV was observed in BM-MSC injected diabetic mice 2 weeks after the injection (Figure 1B). However, 4 weeks after BM-MSC injection, there were no significant differences in sciatic MNCV among the diabetic mice (Figure 1C). These results demonstrate that BM-MSCs improve peripheral nerve conduction in STZ-treated mice. However, the effect of the treatment lasts less than 4 weeks.
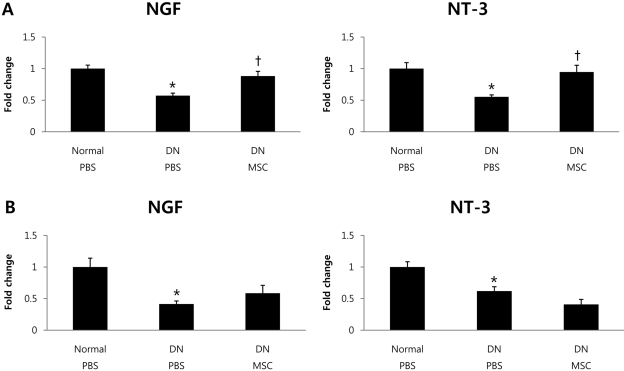
Next, to determine whether nerve conduction increases are associated with alterations in expression levels of NTFs, such as NGF and NT-3, we performed quantitative real-time PCR and examined their mRNA expression in sciatic nerve and femoral muscles at 2 and 4 weeks after BM-MSCs transplantation. Two weeks after transplantation, NGF and NT-3 mRNA levels were significantly decreased in STZ-treated diabetic mice compared with normal mice. The decrease in mRNA expression in STZ-treated mice was ameliorated by BM-MSCs transplantation (Figure 2A). Four weeks after BM-MSCs transplantation, we found slightly increased NGF and NT-3 mRNA levels in some groups, but these changes were not statistically significant (Figure 2B). These results suggest that BM-MSCs improve NTF function in STZ-treated mice. However, in common with MNCV results, the effect of treatment lasted less than 4 weeks.
We measured sciatic MNCV 12 weeks after STZ treatment. Sciatic MNCV was significantly decreased in STZ-treated mice compared with normal mice (Figure 1A). In previous studies, the decreased NGF and NT-3 content led to nerve dysfunction of DN [23,24]. The delayed sciatic MNCV in PBS-injected mice 14 weeks after STZ treatment was significantly ameliorated by BM-MSCs transplantation (Figure 1B).To determine whether the improvement in sciatic nerve conduction is associated with alterations in expression levels of NTFs, we examined NGF and NT-3 mRNA expression in sciatic nerve and femoral muscles. NGF and NT-3 mRNA levels were significantly decreased in diabetic mice compared with normal mice. The decrease in mRNA expression in diabetic mice was ameliorated by BM-MSCs transplantation (Figure 2A).
In the present study, local expression levels of NTFs were decreased in DN mice. Impaired neurotrophic support plays a crucial role in DN, and NTFs have been reported to have a beneficial action on multiple manifestations of diabetes-induced peripheral nerve injury [25]. For example, intrathecal administration of NGF and NT-3 increased myelinated innervation of the dermal footpad of diabetic mice, which suggests that neurotrophic dysfunction plays a crucial role in diabetes-induced impairment of myelinated cutaneous innervations [26]. In the diabetic condition, NGF levels were increased in sympathetic target tissues and decreased in sciatic nerve and sympathetic ganglia [27]. It has been suggested that axonal transport of NGF is impaired in diabetic conditions. Interestingly, improved glucose control restores NGF levels, suggesting that hyperglycemia may be responsible for these abnormalities [28]. Decreased levels of NT-3 in diabetes might contribute to the development of large-fiber sensory neuropathy and later motor neuropathy [15]. Schwann cells are essential for nerve regeneration and they synthesize NTFs such as NGF, BDNF and NT-3, to provide trophic support for regenerating axons [29,30]. Previous studies have demonstrated that BM-MSCs have the capacity to transdifferentiate into cells with a Schwann cell phenotype [31,32]. However, functions of these transdifferentiated Schwann cell-like cells are still questionable. Thus, we need to consider the cellular pathways that mediate interactions between BM-MSCs and Schwann cells.
Additionally, our data suggests that BM-MSCs ameliorated various symptoms of experimental DN through effects on peripheral nerves. However, this effect of the treatment lasts less than 4 weeks. Recent observations indicate that only small numbers of transplanted tissue-specific stem cells engraft into most injured tissues, and they disappear quickly. For example, when bone marrow specific stem cells were injected into the hippocampus in immunodeficient mice, most transplanted cells disappeared within 1 week [33]. Also, after infusion of human MSCs into immunodeficient mice with acute myocardial infarction, engrafted donor cells could not be detected 3 weeks post-injection [34]. For the above reasons, in our study, BM-MSCs might not be more effective on various symptoms of DN at 4 weeks after transplantation.
In this study, we established that local transplantation of BM-MSCs into the muscles around the sciatic nerve reversed the impairment of sciatic MNCV and expression of NTFs in DN mice. It is our hypothesis that reduced expression of NTFs in nerves plays a crucial role in the development and progression of DN, and therefore that therapeutic strategies using BM-MSC transplantation can attenuate DN by supplying NTFs. The present results are the first report that NGF and NT-3 up-regulation in DN are mutually mediated with BM-MSCs. Thus, these results help to elucidate the mechanism by which BM-MSCs function as a cell therapy agent in diabetic neuropathy.
Acknowledgments
This work was supported by the grant for the Future-based Technology Development Program (2010-0020232 to J.S.B.) funded by the National Research Foundation of Korea of the Ministry of Education, Science and Technology, Republic of Korea and the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084065 to H.K.J.).
References
1. Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980; 49(193):95–108. PMID: 7433630.
2. Sampson MJ, Wilson S, Karagiannis P, Edmonds M, Watkins PJ. Progression of diabetic autonomic neuropathy over a decade in insulin-dependent diabetics. Q J Med. 1990; 75(278):635–646. PMID: 2217668.
3. Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000; 43(8):957–973. PMID: 10990072.

4. Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009; 1792(10):931–940. PMID: 19061951.

5. Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003; 27(3):277–324. PMID: 12845152.

6. Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999; 41(Suppl 1):27–34. PMID: 10023126.

7. Yano H, Chao MV. Neurotrophin receptor structure and interactions. Pharm Acta Helv. 2000; 74(2-3):253–260. PMID: 10812966.

8. Rodríguez-Tébar A, Dechant G, Götz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO J. 1992; 11(3):917–922. PMID: 1547788.

9. Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk protooncogene product induced by NGF. Nature. 1991; 350(6314):158–160. PMID: 1706478.

10. Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991; 66(5):967–979. PMID: 1653651.

11. Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc Natl Acad Sci USA. 2001; 98(25):14661–14668. PMID: 11717413.

12. Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004; 43(2):183–191. PMID: 15260955.

13. Xiao J, Wong AW, Willingham MM, Kaasinen SK, Hendry IA, Howitt J, Putz U, Barrett GL, Kilpatrick TJ, Murray SS. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J Neurosci. 2009; 29(13):4016–4022. PMID: 19339597.

14. Tomlinson DR, Fernyhough P, Diemel LT. Neurotrophins and peripheral neuropathy. Philos Trans R Soc Lond B Biol Sci. 1996; 351(1338):455–462. PMID: 8730785.
15. Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997; 46(Suppl 2):S43–S49. PMID: 9285498.

16. Kamiya H, Zhang W, Sima AA. C-peptide prevents nociceptive sensory neuropathy in type 1 diabetes. Ann Neurol. 2004; 56(6):827–835. PMID: 15497155.

17. Wang J, Ding F, Gu Y, Liu J, Gu X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009; 1262:7–15. PMID: 19368814.

18. Cuevas P, Carceller F, Dujovny M, Garcia-Gómez I, Cuevas B, González-Corrochano R, Diaz-González D, Reimers D. Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002; 24(7):634–638. PMID: 12392196.

19. Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg. 2004; 101(5):806–812. PMID: 15540919.

20. Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998; 4(9):1025–1031. PMID: 9734395.

21. Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses. Stem Cells. 2010; 28(2):329–343. PMID: 20014009.

22. Jeong JO, Kim MO, Kim H, Lee MY, Kim SW, Ii M, Lee JU, Lee J, Choi YJ, Cho HJ, Lee N, Silver M, Wecker A, Kim DW, Yoon YS. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009; 119(5):699–708. PMID: 19171856.

23. Fernyhough P, Diemel LT, Hardy J, Brewster WJ, Mohiuddin L, Tomlinson DR. Human recombinant nerve growth factor replaces deficient neurotrophic support in the diabetic rat. Eur J Neurosci. 1995; 7(5):1107–1110. PMID: 7613616.

24. Fernyhough P, Diemel LT, Tomlinson DR. Target tissue production and axonal transport of neurotrophin-3 are reduced in streptozotocin-diabetic rats. Diabetologia. 1998; 41(3):300–306. PMID: 9541170.

25. Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003; 4(9):493–504. PMID: 14636817.

26. Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience. 2007; 145(1):303–313. PMID: 17223273.

27. Hellweg R, Hartung HD. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. J Neurosci Res. 1990; 26(2):258–267. PMID: 2142224.

28. Hellweg R, Wöhrle M, Hartung HD, Stracke H, Hock C, Federlin K. Diabetes mellitus-associated decrease in nerve growth factor levels is reversed by allogeneic pancreatic islet transplantation. Neurosci Lett. 1991; 125(1):1–4. PMID: 1857552.

29. Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004; 10(6):610–616. PMID: 15156204.

30. Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998; 18(7):397–405. PMID: 9880154.

31. Caddick J, Kingham PJ, Gardiner NJ, Wiberg M, Terenghi G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006; 54(8):840–849. PMID: 16977603.

32. Brohlin M, Mahay D, Novikov LN, Terenghi G, Wiberg M, Shawcross SG, Novikova LN. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009; 64(1):41–49. PMID: 19428682.

33. Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005; 102(50):18171–18176. PMID: 16330757.

34. Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007; 354(3):700–706. PMID: 17257581.

Figure 1
Bone marrow-derived mesenchymal stem cells (BM-MSCs) transplantation improved sciatic motor nerve conduction velocity (MNCV) in mice with diabetic neuropathy (DN). (A) 12 weeks after streptozotocin treatment, sciatic MNCV was significantly delayed in streptozotocin-treated mice compared with normal mice (n=10 per group). After measurement of sciatic MNCV, BM-MSCs were transplanted into the muscles percutaneously along the course of the sciatic nerve. (B) 2 weeks after BM-MSC transplantation, a significant improvement of sciatic MNCV occurred in BM-MSCs injected diabetic mice (n=5 per group). (C) 4 weeks after BM-MSCs transplantation, there were no significant differences in sciatic MNCV between any of the diabetic mice groups (n=5 per group). Data represent mean±SEM. Independent t-test, one-way ANOVA, *P<0.05 vs. normal mice; †P<0.05 vs. diabetic mice treated with phosphate-buffered saline (PBS).
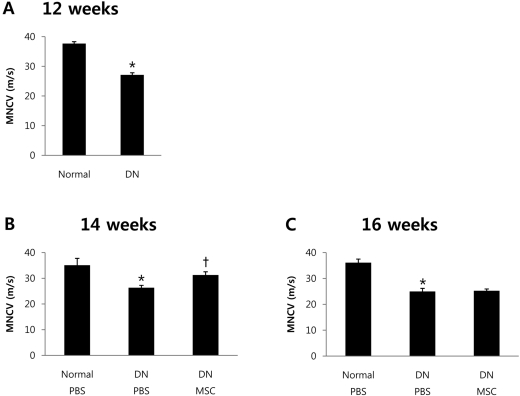
Figure 2
Bone marrow-derived mesenchymal stem cells (BM-MSCs) transplantation increased expression levels of neurotrophic factors (NTFs) in mice with diabetic neuropathy (DN). The mRNA expression levels of NTFs in sciatic nerve and muscle were measured by quantitative real-time polymerase-chain reaction. (A) Two weeks after BM-MSCs transplantation, significant improvement of expression levels of NTFs observed in the BM-MSCs injected diabetic mice (n=5 per group). (B) Four weeks after BM-MSC transplantation, there was no significant difference in expression of NTFs between any of the diabetic mice groups (n=5 per group). Data represent mean±SEM. Independent t-test, one-way ANOVA, *P<0.05 vs. normal mice; †P<0.05 vs. diabetic mice treated with phosphate-buffered saline (PBS).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download