Abstract
The various murine models have contributed to the study of human atopic dermatitis (AD). However limitations of the models involve low reproducibility and long time to develop AD. In an attempt to overcome these limitations and establish an atopic dermatitis murine model, we repeated the application of 2, 4-dinitrochlorobenzene (DNCB) patch in NC/Nga and BALB/c mice, which has advantages in reproduction and cost. For the sensitization, a 1 cm2 gauze-attached patch, where 1% or 0.2% DNCB was periodically attached on the back of NC/Nga and BALB/c mice. To estimate how homologous our model was with human atopic dermatitis, clinical, histological and immunological alterations were evaluated. Both strains showed severe atopic dermatitis, increase in subiliac lymph node weight, mast cells, epidermal hyperplasia and serum IgE levels. Though both exhibited a high IL-4/IFN-γ and IL-4/TNF-β ratio in the expression of mRNA, the shifting of DNCB-treated BALB/c mice was increased to more than double that of NC/Nga mice. These results suggest that our DNCB patched model using BALB/c mice were more suitable than NC/Nga mice in demonstrating the immune response. We anticipate that our novel model may be successfully used for pathogenesis of atopic dermatitis and assessment of therapeutic approaches.
Go to : 
REFERENCES
Hamid Q.., Boguniewicz M.., Leung D.Y.1994. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 94:870–876.

Horsmanheimo L.., Harvima I.T.., Jarvikallio A.., Harvima R.J.., Naukkarinen A.., Horsmanheimo M.1994. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol. 131:348–353.
Akdis C.A.., Akdis M.., Bieber T.., Bindslev-Jensen C.., Boguniewicz M.., Eigenmann P.., Hamid Q.., Kapp A.., Leung D.Y.., Lipozencic J.., Luger T.A.., Muraro A.., Novak N.., Platts-Mills T.A.., Rosenwasser L.., Scheynius A.., Simons F.E.., Spergel J.., Turjanmaa K.., Wahn U.., Weidinger S.., Werfel T.., Zuberbier T.2006. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Allergy. 61:969–987.

Chan L.S.., Robinson N.., Xu L.2001. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 117:977–983.

Dillon S.R.., Sprecher C.., Hammond A.., Bilsborough J.., Rosenfeld-Franklin M.., Presnell S.R.., Haugen H.S.., Maurer M.., Harder B.., Johnston J.., Bort S.., Mudri S.., Kuijper J.L.., Bukowski T.., Shea P.., Dong D.L.., Dasovich M.., Grant F.J.., Lockwood L.., Levin S.D.., LeCiel C.., Waggie K.., Day H.., Topouzis S.., Kramer J.., Kuestner R.., Chen Z.., Foster D.., Parrish-Novak J.., Gross J.A.2004. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 5:752–760.

Friedmann P.S.2006. Contact sensitisation and allergic contact dermatitis: immunobiological mechanisms. Toxicol Lett. 162:49–54.

Guo T.L.., Zhang X.L.., Leffel E.K.., Peachee V.L.., Karrow N.A.., Germolec D.R.., White, K.L. Jr.2002. Differential stimulation of IgE production, STAT activation and cytokine and CD86 expression by 2,4-dinitrochlorobenzene and trimellitic anhydride. J Appl Toxicol. 22:397–403.

Gustafsson D.., Sjoberg O.., Foucard T.2000. Development of allergies and asthma in infants and young children with atopic dermatitis–a prospective followup to 7 years of age. Allergy. 55:240–245.
Hamid Q.., Boguniewicz M.., Leung D.Y.1994. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 94:870–876.

Horsmanheimo L.., Harvima I.T.., Jarvikallio A.., Harvima R.J.., Naukkarinen A.., Horsmanheimo M.1994. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol. 131:348–353.
Inoue Y.., Isobe M.., Shiohara T.., Goto Y.., Hayashi H.2002. Protective and curative effects of topically applied CX-659S, a novel diaminouracil derivative, on chronic picryl chloride-induced contact hypersensitivity responses. Br J Dermatol. 147:675–682.

Jin H.., He R.., Oyoshi M.., Geha R.S.2009. Animal models of atopic dermatitis. J Invest Dermatol. 129:31–40.

Kang J.S.., Lee K.., Han S.B.., Ahn J.M.., Lee H.., Han M.H.., Yoon Y.D.., Yoon W.K.., Park S.K.., Kim H.M.2006. Induction of atopic eczema/dermatitis syndrome-like skin lesions by repeated topical application of a crude extract of Dermatophagoides pteronyssinus in NC/Nga mice. Int Immunopharmacol. 6:1616–1622.

Kim E.C.., Lee H.S.., Kim S.K.., Choi M.S.., Lee S.., Han J.B.., An H.J.., Um J.Y.., Kim H.M.., Lee N.Y.., Bae H.., Min B.I.2008. The bark of Betula platyphylla var. japonica inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. J Ethnopharmacol. 116:270–278.
Kimber I.., Basketter D.A.., Berthold K.., Butler M.., Garrigue J.L.., Lea L.., Newsome C.., Roggeband R.., Steiling W.., Stropp G.., Waterman S.., Wiemann C.2001. Skin sensitization testing in potency and risk assessment. Toxicol Sci. 59:198–208.

Kitagaki H.., Fujisawa S.., Watanabe K.., Hayakawa K.., Shiohara T.1995. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol. 105:749–755.

Kitagaki H.., Ono N.., Hayakawa K.., Kitazawa T.., Watanabe K.., Shiohara T.1997. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 159:2484–2491.
Lee H.S.., Kim S.K.., Han J.B.., Choi H.M.., Park J.H.., Kim E.C.., Choi M.S.., An H.J.., Um J.Y.., Kim H.M.., Min B.I.2006. Inhibitory effects of Rumex japonicus Houtt. on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Br J Dermatol. 155:33–38.
Leung D.Y.., Boguniewicz M.., Howell M.D.., Nomura I.., Hamid Q.A.2004. New insights into atopic dermatitis. J Clin Invest. 113:651–657.

Man M.Q.., Hatano Y.., Lee S.H.., Man M.., Chang S.., Feingold K.R.., Leung D.Y.., Holleran W.., Uchida Y.., Elias P.M.2008. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 128:79–86.

Matsuda H.., Watanabe N.., Geba G.P.., Sperl J.., Tsudzuki M.., Hiroi J.., Matsumoto M.., Ushio H.., Saito S.., Askenase P.W.., Ra C.1997. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 9:461–466.

Navi D.., Saegusa J.., Liu F.T.2007. Mast cells and immunological skin diseases. Clin Rev Allergy Immunol. 33:144–155.

Nomura I.., Goleva E.., Howell M.D.., Hamid Q.A.., Ong P.Y.., Hall C.F.., Darst M.A.., Gao B.., Boguniewicz M.., Travers J.B.., Leung D.Y.2003. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 171:3262–3269.

Novak N.2009. New insights into the mechanism and management of allergic diseases: atopic dermatitis. Allergy. 64:265–275.

Novak N.., Bieber T.2005. The role of dendritic cell subtypes in the pathophysiology of atopic dermatitis. J Am Acad Dermatol. 53:S171–176.

Shiohara T.., Hayakawa J.., Mizukawa Y.2004. Animal models for atopic dermatitis: are they relevant to human disease? J Dermatol Sci. 36:1–9.

Spergel J.M.., Mizoguchi E.., Brewer J.P.., Martin T.R.., Bhan A.K.., Geha R.S.1998. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 101:1614–1622.

Sugiura K.., Shamoto M.., Sakamoto N.., Shinzato M.., Osada A.., Sugiura M.., Hayakawa R.., Kato Y.2003. It is true that, when Langerhans cells migrate from the skin to the lymph node, they are transported via lymph vessels. Dermatology. 206:222–224.

Sunada Y.., Nakamura S.., Kamei C.2008. Effect of Lactobacillus acidophilus strain L-55 on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 8:1761–1766.

Tanaka T.., Tsutsui H.., Yoshimoto T.., Kotani M.., Matsumoto M.., Fujita A.., Wang W.., Higa S.., Koshimoto T.., Nakanishi K.., Suemura M.2001. Interleukin-18 is elevated in the sera from patients with atopic dermatitis and from atopic dermatitis model mice, NC/Nga. Int Arch Allergy Immunol. 125:236–240.

Taniguchi Y.., Kohno K.., Inoue S.., Koya-Miyata S.., Okamoto I.., Arai N.., Iwaki K.., Ikeda M.., Kurimoto M.2003. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 3:1313–1324.

Theiner G.., Gessner A.., Lutz M.B.2006. The mast cell mediator PGD2 suppresses IL-12 release by dendritic cells leading to Th2 polarized immune responses in vivo. Immunobiology. 211:463–472.

Wollenberg A.., Klein E.2007. Current aspects of innate and adaptive immunity in atopic dermatitis. Clin Rev Allergy Immunol. 33:35–44.

Yamanaka K.., Tanaka M.., Tsutsui H.., Kupper T.S.., Asahi K.., Okamura H.., Nakanishi K.., Suzuki M.., Kayagaki N.., Black R.A.., Miller D.K.., Nakashima K.., Shimizu M.., Mizutani H.2000. Skin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18. J Immunol. 165:997–1003.

Go to : 
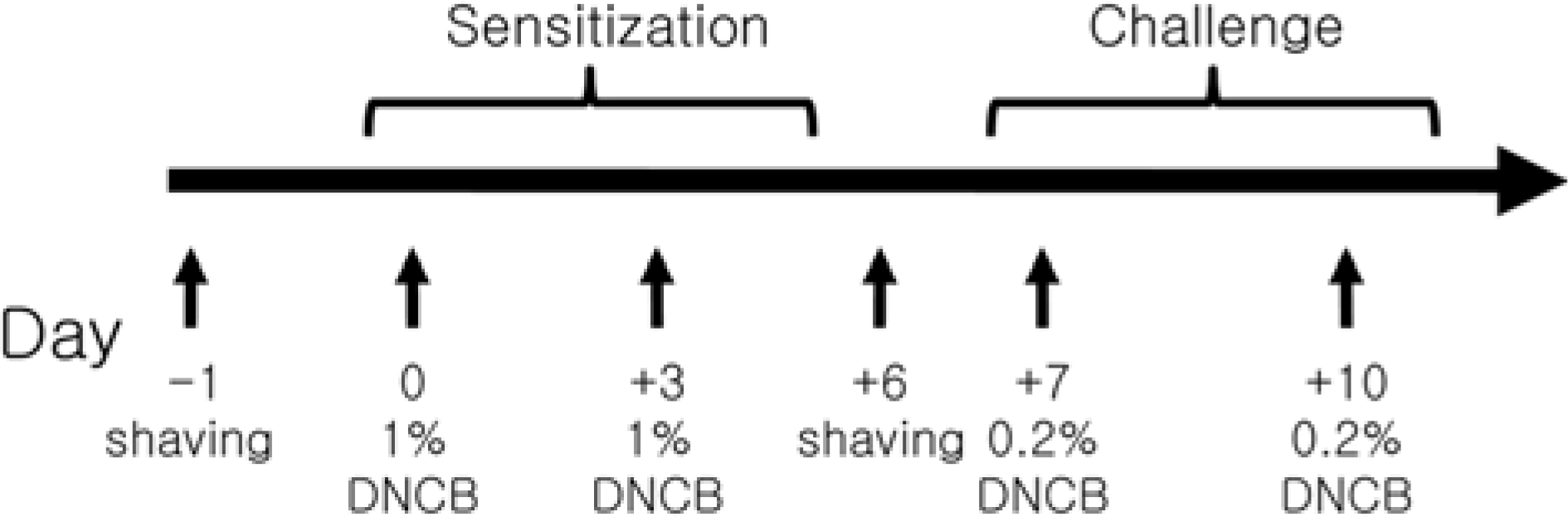 | Figure 1.Schematic diagram of the study protocol. The back of NC/Nga mice and BALB/c were shaved with electric clipper and depilatory cream. 1 cm2-gauze treated 0.1 mL of 2% DNCB and vehicle was placed on a patch, which was attached to the mice twice a week for sensitization. After sensitization, 0.1 mL of 0.2% DNCB and vehicle were used to challenge the animals as the previous sensitization. |
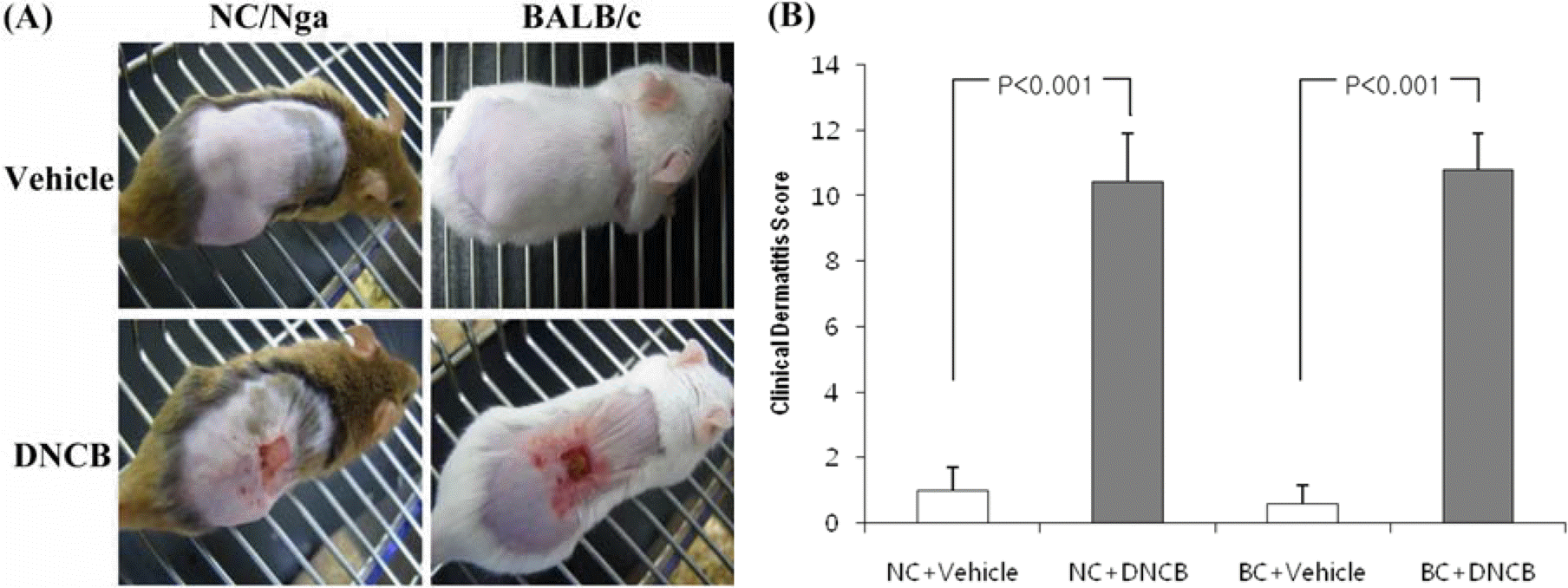 | Figure 2.(A) Pictures of the clinical observations on the back of mice. (B) Evaluation of clinical dermatitis induced by DNCB and vehicle. The evaluation of clinical dermatitis was based on the areas of gauze which delivered the DNCB and vehicle. Each column shows the mean±SD of 5 mice. |
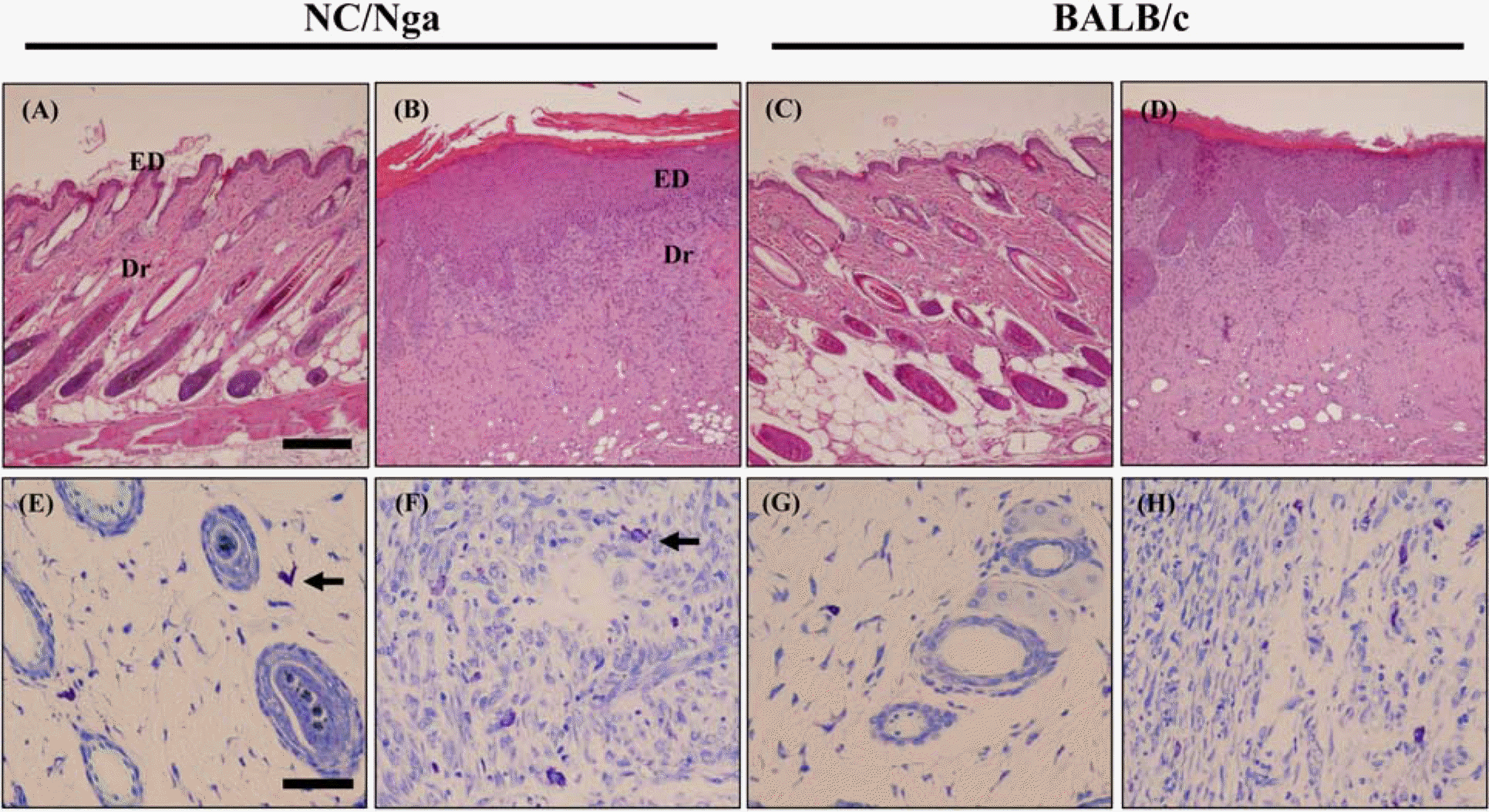 | Figure 3.Histopathological alterations by DNCB and vehicle. Skins of mice were stained with hematoxylin & eosin (×100, A-D) and toluindine blue (×400, E-H). (A, C, E, G) are vehicle-treated groups and (B, D, F, H) are DNCB-treated groups. ED, epidermis; Dr, dermis; arrow, mast cells. Bar in ×100=200 µm, Bar in ×400=50 µm. |
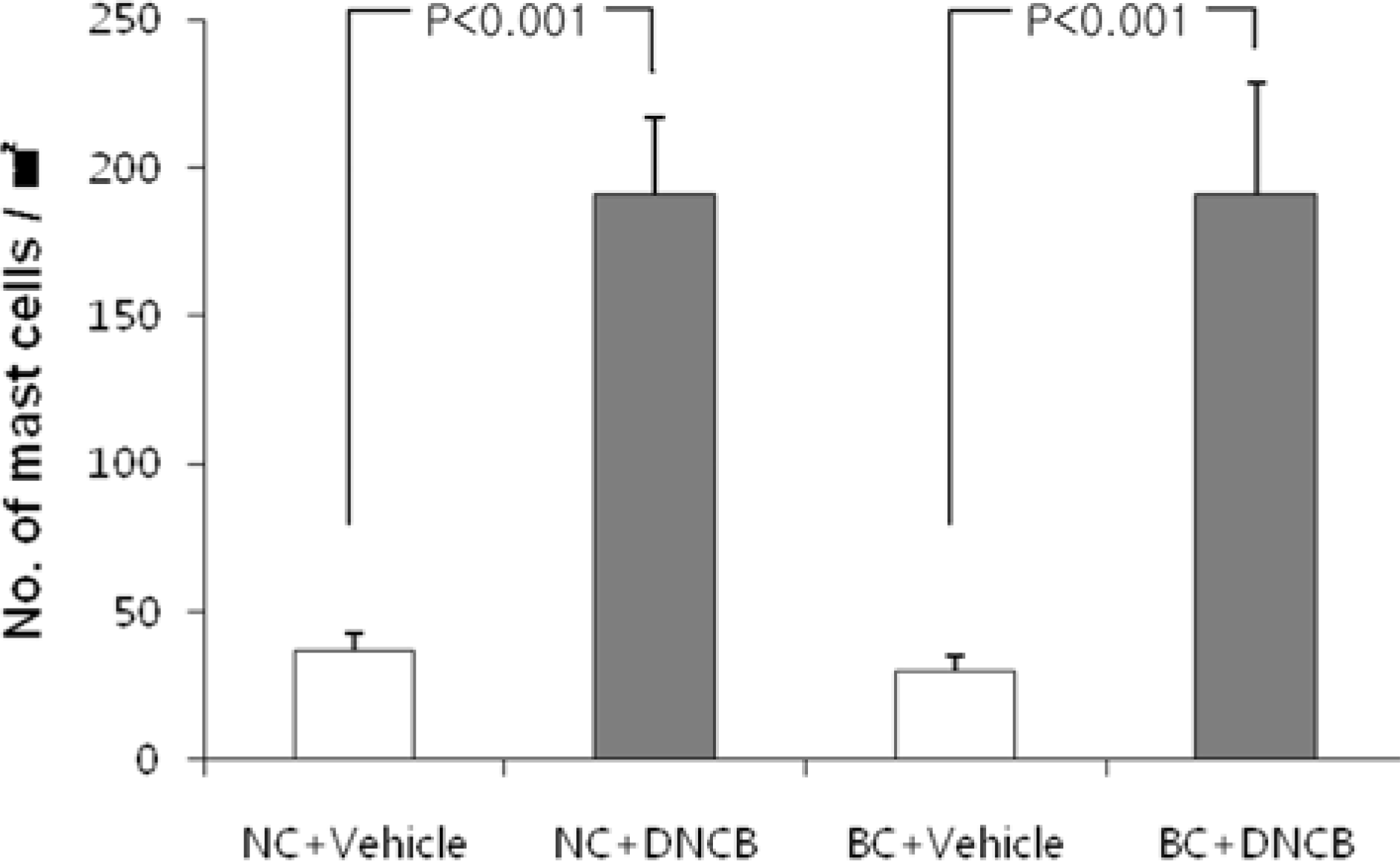 | Figure 4.Quantification of mast cells in dorsal skin of mice treated with DNCB and vehicle. Skin of mice were stained with toluidine blue and the numbers of mast cell in the dermis of the skin were quantitatively analyzed in 5 high power fields (×400) by a computerized image analyzer. Each column shows the mean±SD of 5 mice. |
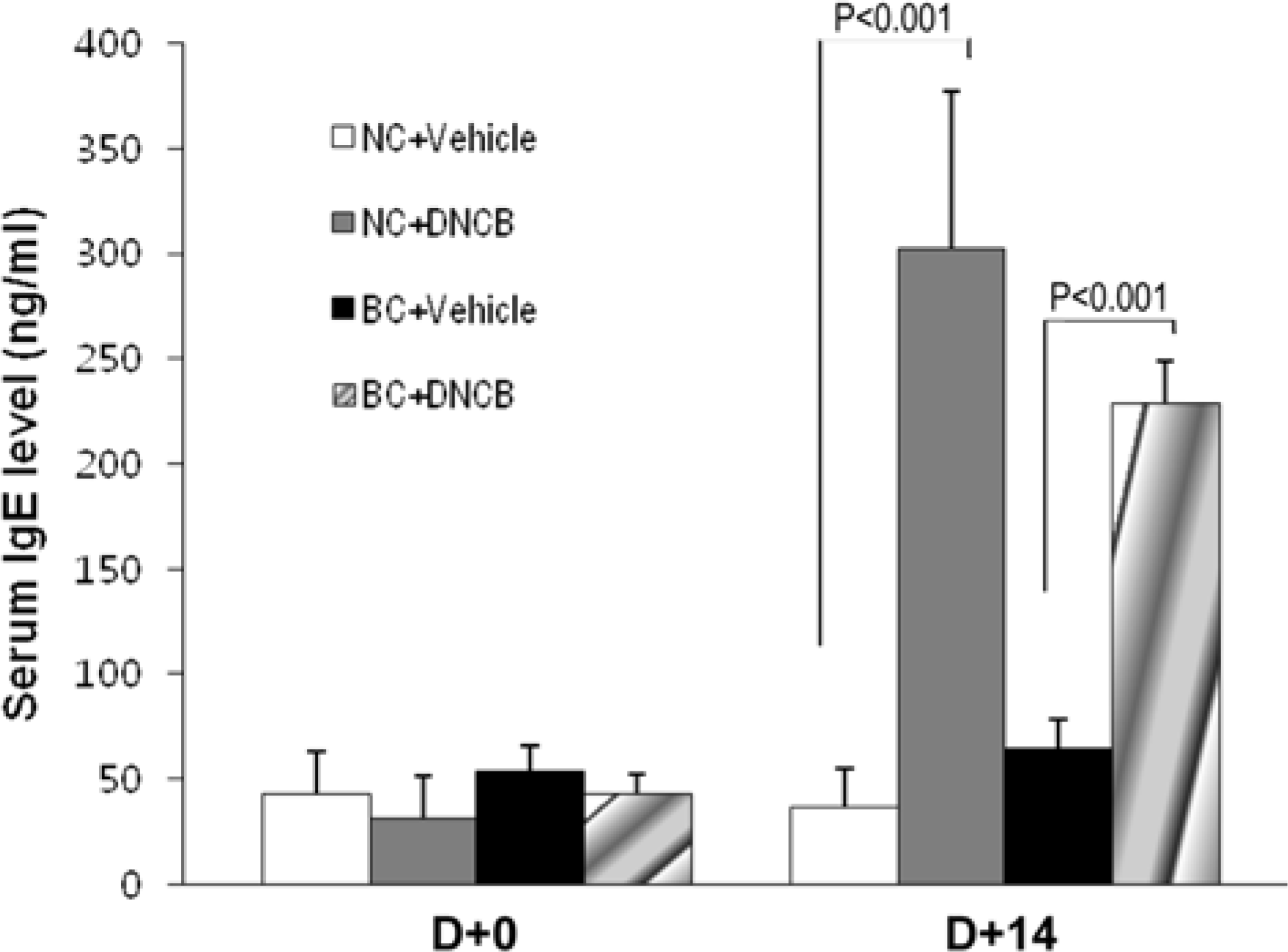 | Figure 5.Changes in serum total IgE levels. Blood samples were collected from retro-obital venous plexus before treatment with DNCB and at 4 days after the last treatment. Serum IgE was quantified using ELISA. Each column shows the mean± SD of 4 mice. |
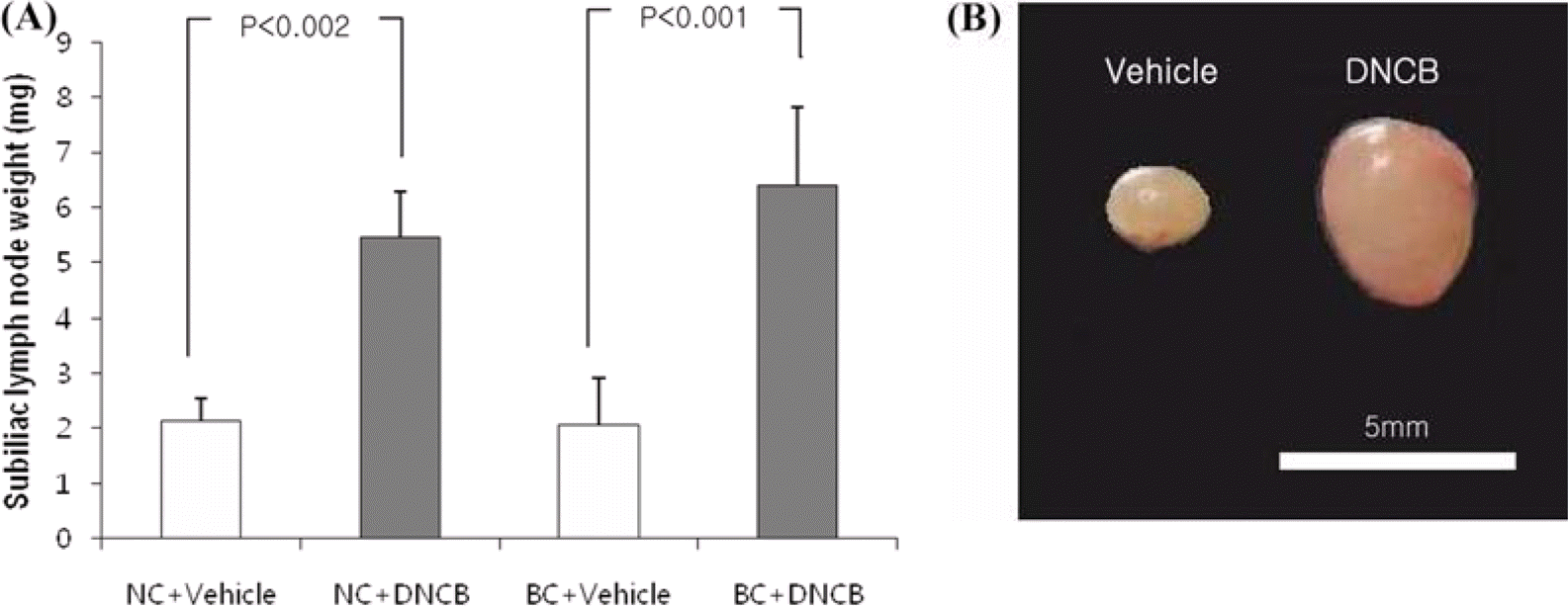 | Figure 6.Changes in subiliac lymph node weight induced by DNCB and vehicle. The subiliac lymph node approximates to the site, treated by DNCB and vehicle. The weights of subiliac lymph node were measured 8 days after the last treatment. Each column shows the mean±SD of 5 mice. |
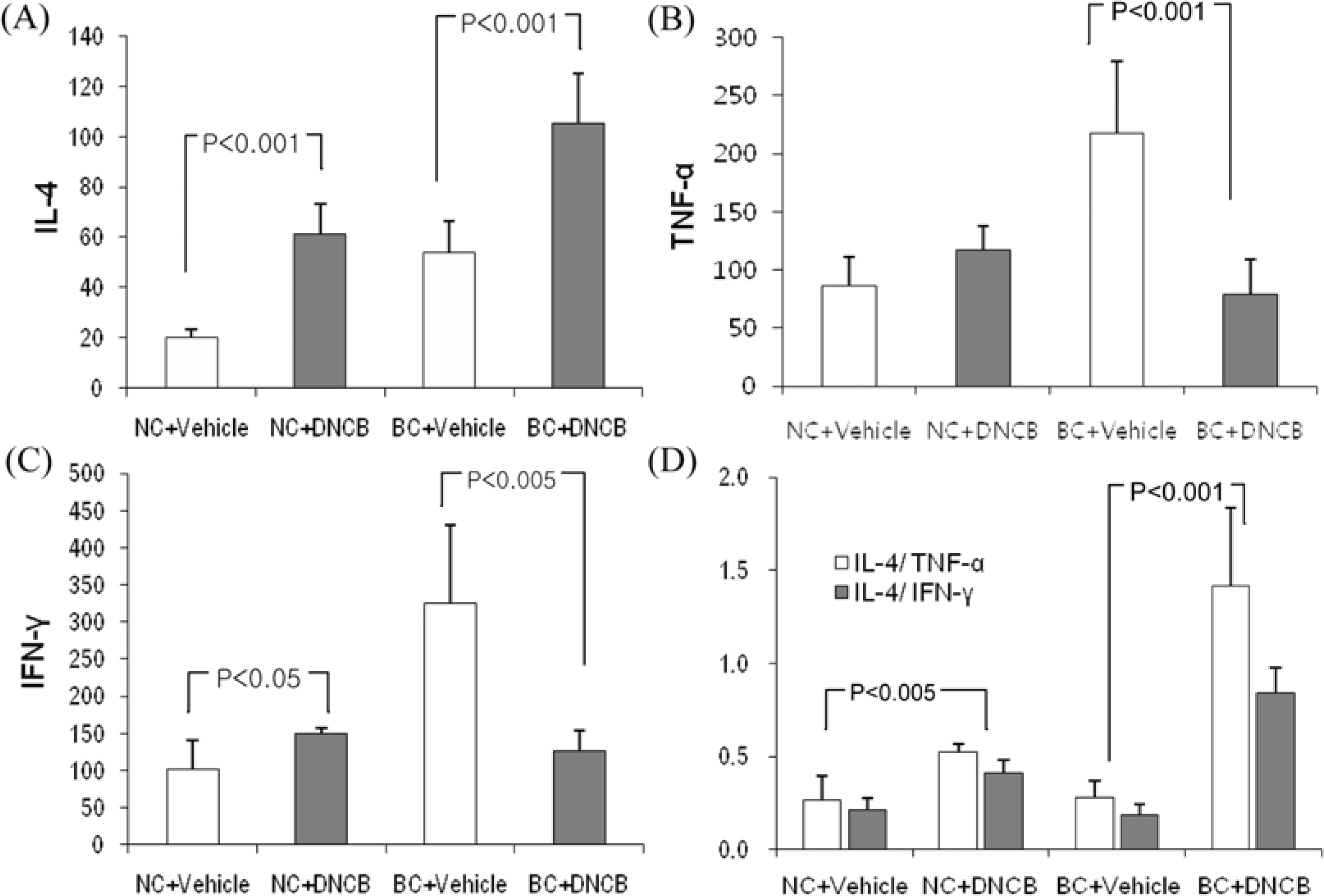 | Figure 7.Expression of IL-4, IFN-γ and TNF-α mRNA levels in skin painted repeatedly with DNCB and vehicle. Total mRNA was extracted from skin and reverse-transcribed as described in materials and methods. IL-4 (A), TNF-α (B) and IFN-γ (C) mRNA expression were normalized with β-actin. (D) IL-4/TNF-α (white bars) and IL-4/IFN-γ mRNA (black bars). Each column shows the mean±SD of 5 mice. |
Table 1.
cytokine-specific primer pair sequences used in RT-PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download