Abstract
Spatial frequency and direction tuning to drifting sinusoidal gratings are intrinsic properties of neurons in visual cortex neurons in areas 17 and 18. To investigate the stability of these tuning properties during visual stimulation in anesthetized cats, the temporal dynamics of spatial frequency and direction tuning were analyzed in every 0.1 sec. The responses of cortical neurons (n=109) as a function of spatial frequency as well as direction at a particular velocity for 1 second were measured and plotted as a contour plot. Five parameters from this plot were extracted: optimum response, preferred direction (direction that showed the optimum response), optimum spatial frequency (spatial frequency that showed the optimum response), direction tuning width (the difference between the highest and lowest directions to which the cell was at least half as responsive as it was to its optimum direction) and spatial frequency bandwidth (the difference between the highest and lowest frequencies to which the cell was at least half as responsive as it was to its optimum frequency). Then, this contour plot was further analyzed in every 0.1 sec to investigate whether these five parameters were changed or not during the course of stimulation. These parameters were plotted along the time (0.1 sec step) and a line of fit was found. Both spatial frequency and direction tuning properties were not changed in most of the cells. This suggests that both direction and spatial frequency tuning properties are stable during drifting sinusoidal gratings’ stimulation.
Go to : 
REFERENCES
Bredfeldt C.E.., Ringach D.L.2002. Dynamics of spatial frequency tuning in macaque V1. J. Neurosci. 22(5):1976–1984.

Campbell F.W.., Cleland B.G.., Cooper G.F.., Enroth-Cugell C.1968. The angular selectivity of visual cortical cells to moving gratings. J. Physiol. 198(1):237–250.

Campbell F.W.., Cooper G.F.., Enroth-Cugell C.1969. The spatial selectivity of the visual cells of the cat. J. Physiol.
Celebrini S.., Thorpe S.., Trotter Y.., Imbert M.1993. Dynamics of orientation coding in area V1 of the awake primate. Vis. Neurosci. 10(5):811–825.

Chen G.., Dan Y.., Li C.Y.2005. Stimulation of nonclassical receptive field enhances orientation selectivity in the cat. J. Physiol. 564(1):233–243.

De Valois R.L.., Albrecht D.G.., Thorell L.G.1982. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 22(5):545–559.

Frazor R.A.., Albrecht D.G.., Geisler W.S.., Crane A.M.2004. Visual cortex neurons of monkeys and cats: temporal dynamics of the spatial frequency response function. J. Neurophysiol. 91(6):2607–2627.

Tusa R.J.., Palmer L.A.., Rosenquist A.C.1978. The retinotopic organization of area 17 (striate cortex) in the cat. J. Comp. Neurol. 177(2):213–235.

Volgushev M.., Vidyasagar T.R.., Pei X.1995. Dynamics of the orientation tuning of postsynaptic potentials in the cat visual cortex. Vis. Neurosci. 12(4):621–628.

Gillespie D.C.., Lampl I.., Anderson J.S.., Ferster D.2001. Dynamics of the orientation-tuned membrane potential response in cat primary visual cortex. Nature Neurosci. 4(10):1014–1019.

Hammond P.., Pomfrett C.J.1990. Influence of spatial frequency on tuning and bias for orientation and direction in the cat's striate cortex. Vision Res. 30(3):359–369.

Jones J.P.., Stepnoski A.., Palmer L.A.1987. The two-dimensional spectral structure of simple receptive fields in cat striate cortex. J. Neurophysiol. 58(6):1212–1232.

Kim J.N.., Leem M.A.2008. Temporal coding of the spatial frequency and direction tuning in cat cortical areas 17 and 18. Soc. for Neurosci. 459.8.
Maffei L.., Fiorentini A.1973. The visual cortex as a spatial frequency analyzer. Vision Res. 13(7):1255–1267.
Malone B.J.., Ringach D.L.2008. Dynamics of tuning in the Fourier domain. J. Neurophysiol. 100(1):239–248.

Mazer J.A.., Vinje W.E.., McDermott J.., Schiller P.H.., Gallant J.L.2002. Spatial frequency and orientation tuning dynamics in area V1. Proc. Natl. Acad. Sci. U S A. 99(3):1645–1650.

Movshon J.A.., Thompson I.D.., Tolhurst D.J.1978. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J. Physiol. 283(1):101–120.

Nishimoto S.., Arai M.., Ohzawa I.2005. Accuracy of subspace mapping of spatiotemporal frequency domain visual receptive fields. J. Neurophysiol. 93(6):3524–3536.

Park Y.., Kim J.N.2004. Spatial frequency and velocity tunings of neurons in areas 17 and 18 of the cat using a 100 microelectrode array. Korean J. Lab. Anim. Sci. 20(1):26–30.
Ringach D.L.., Hawken M.J.., Shapley R.1997. Dynamics of orientation tuning in macaque primary visual cortex. Nature. 387(6630):281–284.

Ringach D.L.., Hawken M.J.., Shapley R.2003. Dynamics of orientation tuning in macaque V1: the role of global and tuned suppression. J. Neurophysiol. 90(1):342–352.
Rousche P.J.., Normann R.A.1992. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann. Biomed. Eng. 20(4):413–422.

Schummers J.., Cronin B.., Wimmer K.., Stimberg M.., Martin R.., Obermayer K.., Koerding K.., Sur M.2007. Dynamics of orientation tuning in cat v1 neurons depend on location within layers and orientation maps. Front Neurosci. 1(1):145–159.

Shapley R.., Hawken M.., Ringach D.L.2003. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron. 38(5):689–699.

Shapley R.., Hawken M.., Xing D.2007. The dynamics of visual responses in the primary visual cortex. Prog. Brain Res. 165(1):21–32.

Sharon D.., Grinvald A.2002. Dynamics and constancy in cortical spatiotemporal patterns of orientation processing. Science. 295(5554):512–515.

Tolhurst D.J.., Movshon J.A.1975. Spatial and temporal contrast sensitivity of striate cortical neurones. Nature. 257(5528):674–675.

Go to : 
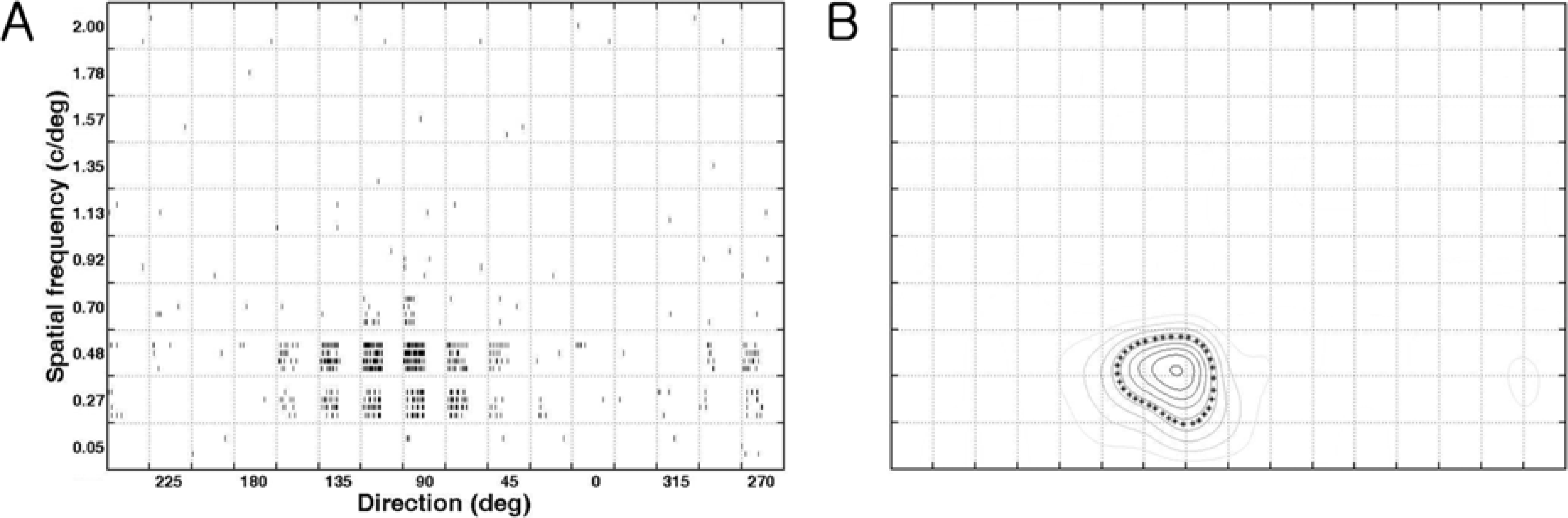 | Figure 1.Quantification of direction/spatial frequency tuning plot (cell number: 0709_c65_u3_v5). The stimuli were sinusoidal gratings with 10 spatial frequencies (the y-axis) in 16 directions (22.5 deg interval, the x-axis) at a particular velocity (4.6 deg/sec). (A). Raster plots of responses of a neuron in a joint direction and spatial frequency domain. Raster plots show responses to 160 different stimuli (combinations of 10 spatial frequencies and 16 directions). (B). The contour plot in a joint direction and spatial frequency domain. The contour at the 50 % of the maximum response, at the optimal direction (x-axis) and at the optimal spatial frequency (y-axis), is indicated by asterisks. Therefore, five values were calculated (optimal response: 45 impulses/sec, optimal direction: 104.6 deg, tuning width: 47.4 deg, optimal spatial frequency: 0.46 c/deg, bandwidth: 0.36 c/deg) from this contour plot after measuring two more values including horizontal distance (i.e. tuning width) and vertical distance (i.e. bandwidth) of the boundary. |
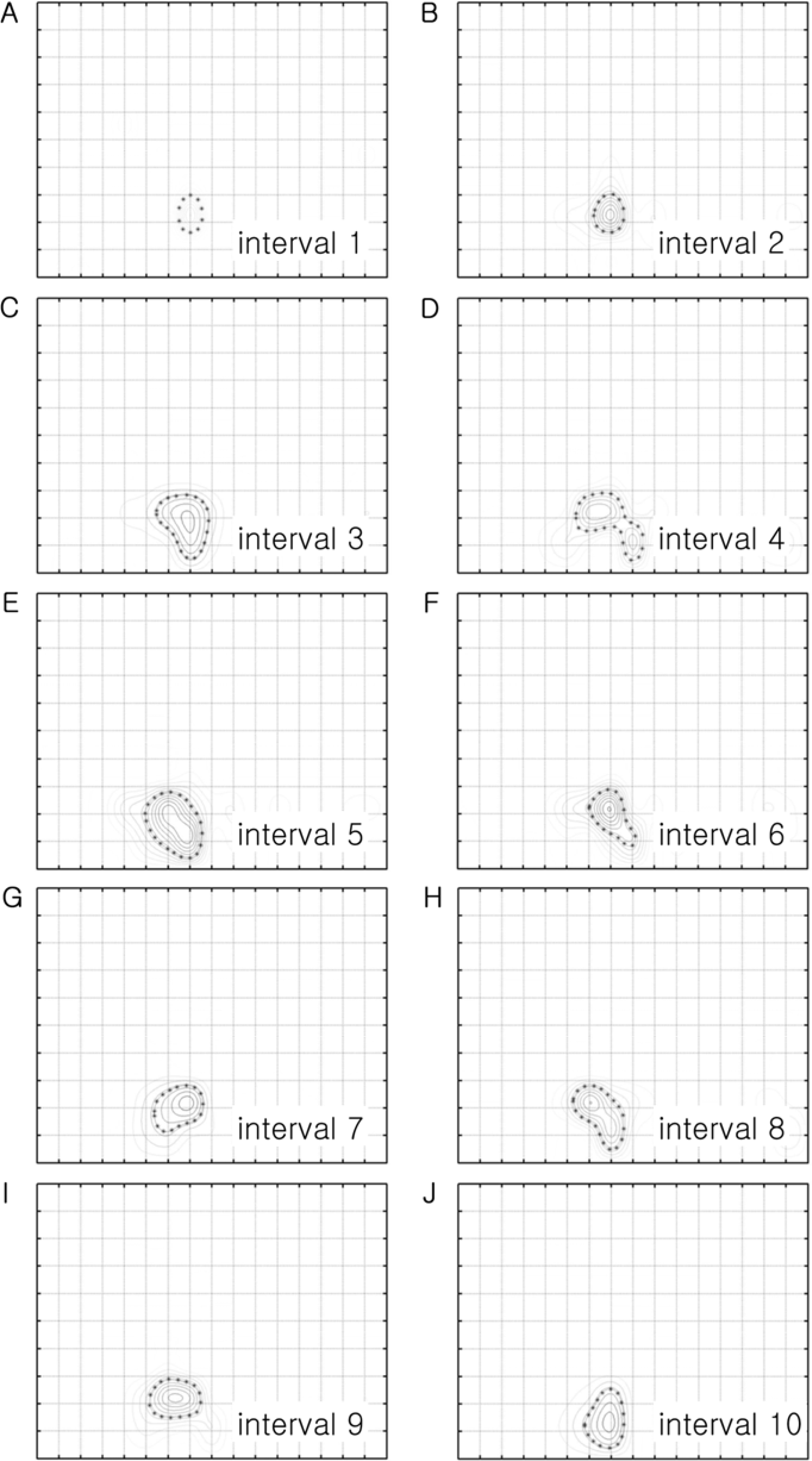 | Figure 2.Temporal analysis of the response of the cell shown in Figure 1. The contour plot in Figure 1B was analyzed in every 100 msec, from the beginning of the stimulation to the end of stimulation, to draw 10 contour plots. Figure 2A is the contour plot at the 1st interval time (between 0 and 0.1 sec) and Figure 2J is the contour plot at the 10th interval time (between 0.9-1.0 sec). The shapes of the boundary at the 50% of the optimal response are slightly different among them. But, it is generally a round shape even though it shows two peaks in Figure 2D (i.e. the 4th interval time). The size of the boundary becomes bigger at the 3rd interval and becomes similar up to the 10th interval. |
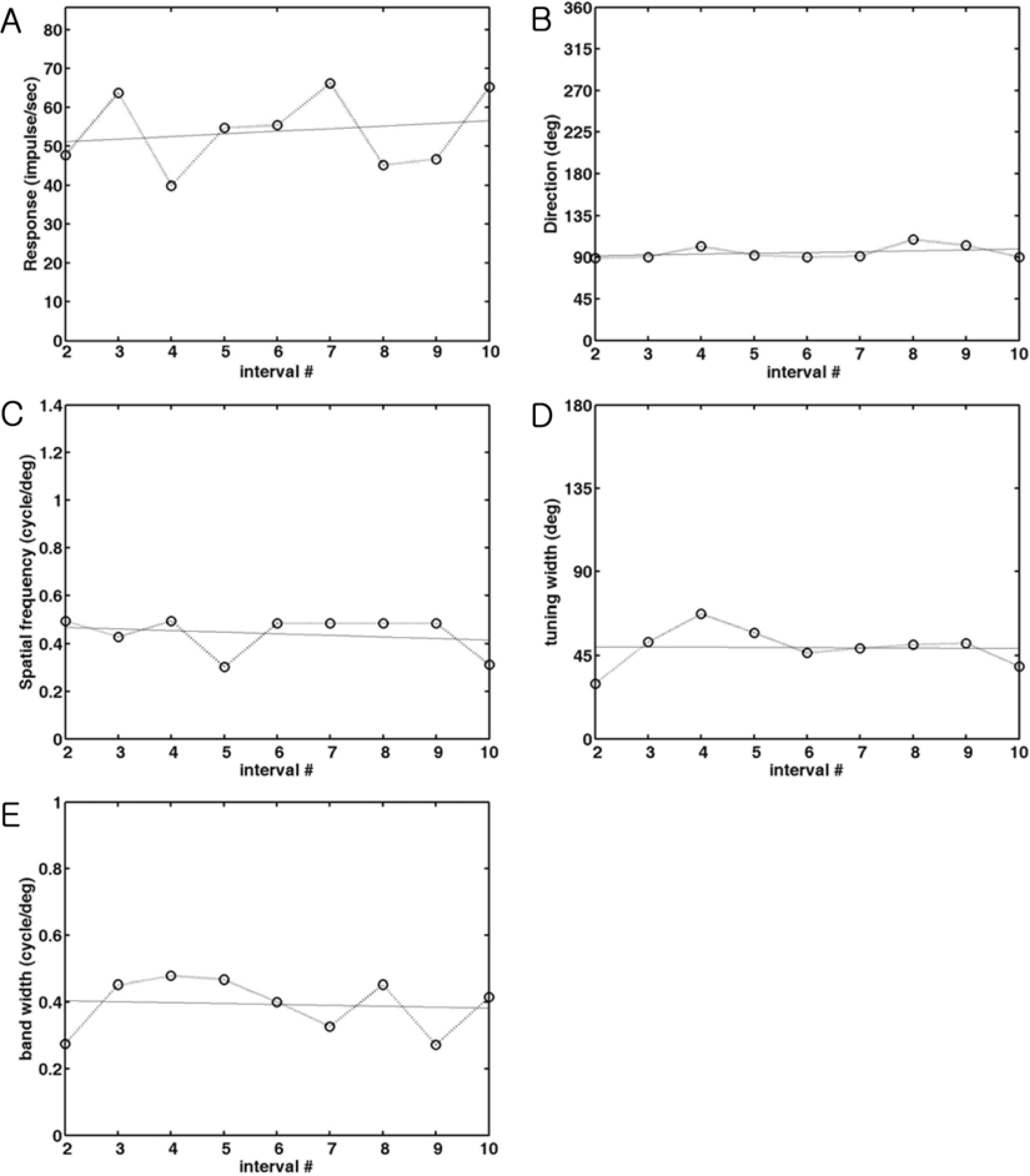 | Figure 3.The changes of parameters during stimulation. Each parameter measured in every 0.1 sec is plotted against the number of interval time between the 2nd interval and 10th interval to quantify the change. The parameters at the 1st interval are not included because the peak at the 1st interval is generally weak. A line of fit was found to see the increase or decrease of these parameters during stimulation and was also drawn in the same graph. The meaning of the slope of the fitted line is the changes of values from the 2nd interval to the 10th interval time. Positive values indicate the increase of parameter, whereas negative values indicate the decrease of parameter. (A). The change of optimal response. The optimal response measured in each interval is plotted as a function of time. The slope of a fitting line is 0.07 which means not much change during stimulation. (B). The change of optimal direction. The optimal direction measured in each interval is plotted as a function of time. The slope of a fitting line is 0.93 which means 10 degree change during stimulation. (C). The change of optimal spatial frequency. The optimal spatial frequency measured in each interval is plotted as a function of time. The slope of a fitting line is 0.007, which means 0.63 cycle/degree change during stimulation. (D). The change of tuning width. The direction tuning width measured in each interval is plotted as a function of time. The slope of a fitting line is -0.10, which means 1 degree change during stimulation. (E). The change of spatial frequency bandwidth. The spatial frequency bandwidth at each interval is plotted as a function of time. The slope of a fitting line is 0.003, which means 0.03 cycle/degree change during stimulation. |
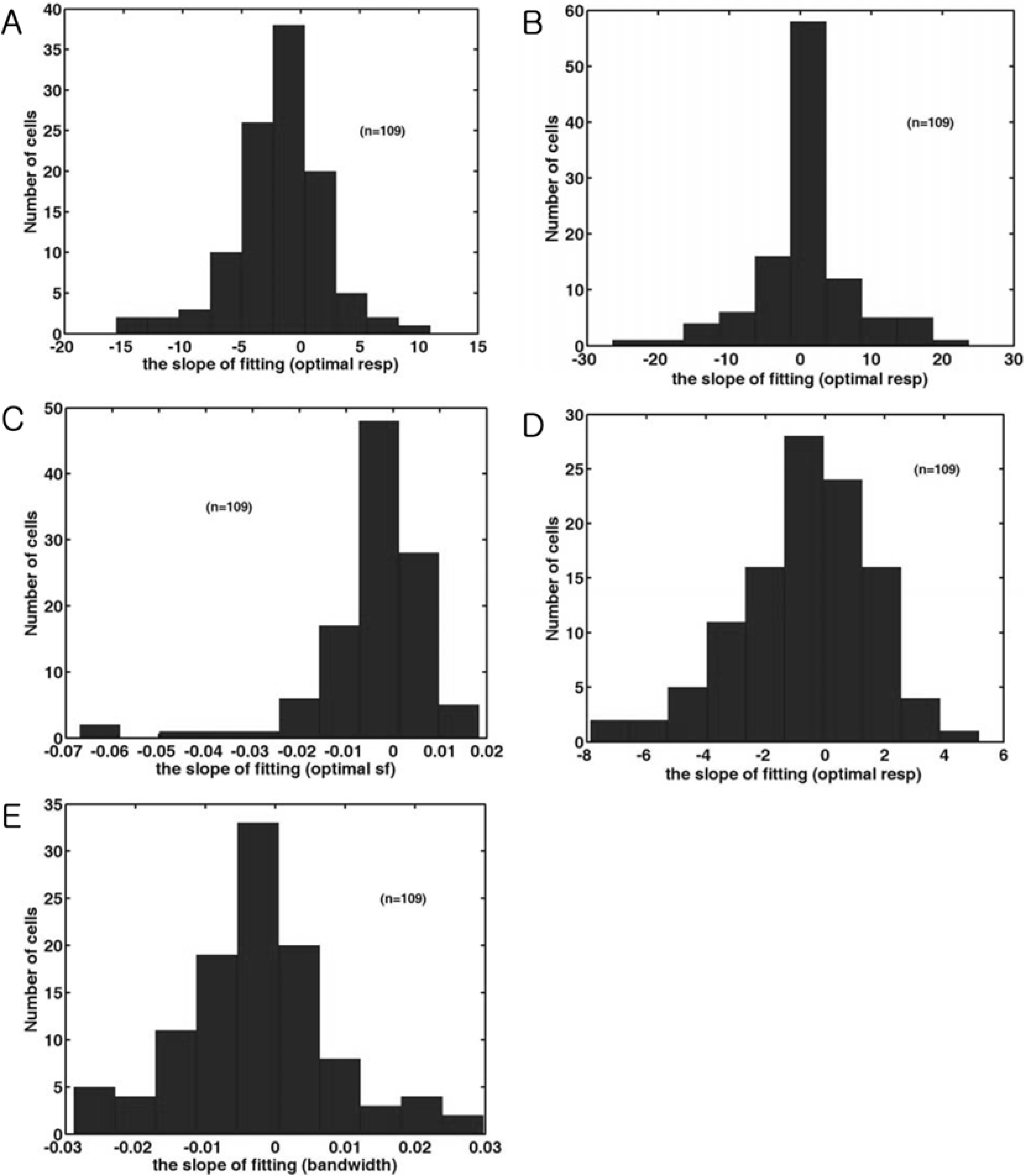 | Figure 4.The distribution of the slope of fitting line of five parameters. (A). Histogram of the slope of fitting line in the optimal response of the time-averaged response of each cell. (B). Histogram of the slope of fitting line in the optimal direction of the time-averaged response of each cell. (C). Histogram of the slope of fitting line in the optimal spatial frequency of the time-averaged response of each cell. Positive numbers indicate a shift from low to high spatial frequencies, whereas negative numbers indicate a shift from high to low spatial frequencies. (D). Histogram of the slope of fitting line in the direction tuning width of the time-averaged response of each cell. A positive value indicates that the tuning width is increased. (E). Histogram of the spatial frequency bandwidth of the time-averaged response of each cell. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download