Abstract
Pressure overload diseases such as valvular stenosis and systemic hypertension morphologically manifest in patients as cardiac concentric hypertrophy. Preventing cardiac remodeling due to increased pressure overload is important to reduce the morbidity and mortality. A recent clinical study has shown that carvedilol has beneficial effects on the survival rate of patients with heart failure. This may be due to the actions of carvedilol such as β-adrenoceptor blockade and the α1-adrenergic receptor blockade effects. Therefore, we investigated whether carvedilol can reverse preexisting cardiac hypertrophy and we compared the effects of racemic carvedilol and the carvedilol enantiomers. Cardiac hypertrophy was induced in rats by suprarenal transverse abdominal aortic constriction (AC). Fifteen weeks after AC surgery, concentric hypertrophy was identified in the AC group by performing echocardiography. Low dose S- and SR-carvedilol (2 mg/kg/day), which were orally administered for three weeks, caused significant regression of the cardiac hypertrophy, and this most significantly occurred in the rats that received S-carvedilol. However, R-carvedilol did not reduce cardiac hypertrophy. Regression of cardiac hypertrophy by carvedilol was confirmed on the echocardiograms and electrocardiograms. These results suggest that carvedilol could reverse the development of leftventricular concentric hypertrophy that is induced by pressure overload. S-carvedilol is proposed to be superior to SR-and R-carvedilol as a beneficial treatment for cardiac hypertrophy.
REFERENCES
Baba H.A.., Iwai T.., Bauer M.., Irlbeck M.., Schmid K.W.., Zimmer H.G.1999. Differential effects of angiotensin II receptor blockade on pressure-induced left ventricular hypertrophy and fibrosis in rats. J. Mol. Cell. Cardiol. 31:445–455.

Bartsch W.., Sponer G.., Strein K.., Muller-Beckmann B.., Kling L.., Bohm E.., Martin U.., Borbe H.O.1990. Pharmacological characteristics of the stereoisomers of carvedilol. Eur. J. Clin. Pharmacol. 38 (Suppl. 2), S104-S107.
Bohm M.., Laragh J.H.., Zehender M.1992. From Hypertension to Heart Failure. pp. 177-205, Springer, Berlin.
Bril A.., Slivjak M.., Dimartino M.J.1992. Cardioprotective effects of carvedilol, a novel β-adrenoceptor antagonist with vasodilation properties, in anaesthetized minipigs: comparison with propranolol. Cardiovasc. Res. 26:518–525.
Bristow M.R.., Gilbert E.M.., Abraham W.T.., Adams K.F.., Fowler M.B.., Hershberger R.E.., Kubo S.H.., Narahara K.A.., Ingersoll H.., Krueger S.., Young S.., Shusterman N.1996. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 94:2807–2816.

Cohn J.N.., Fowler M.B.., Bristow M.R.., Colucci W.S.., Gilbert E.M.., Kinhal V.., Krueger S.K.., Lejemtel T.., Narahara K.A.., Packet M.., Young S.T.., Holcslaw T.L.., Lukas M.A.1997. Safety and efficacy of carvedilol in severe heart failure. J. Card. Failure. 3:173–179.

Colucci W.S.., Wynne J.., Holman B.L.., Braunwald E.1980. Longterm therapy of heart failure with prazosin: a randomized double blind trial. Am. J. Cardiol. 45:337–344.

Dupont A.G.1990. Effects of carvedilol on renal function. Eur. J. Clin. Pharmacol. 38 (Suppl. 2), S96-S100.
Esposito G.., Rapacciuolo A.., Naga Prasad S.V.., Takaoka H.., Thomas S.A.., Koch W.J.., Rockman H.A.2002. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 105:85–92.

Fujimaki M.1992. Stereoselective disposition and tissue distribution of carvedilol enantiomers in rats. Chirality. 4(3):148–154.

Gilbert E.M.., Olsen S.L.., Renlund D.G.., Bristow M.R.1993. β-adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 71, 23C-29C.
Gregorini L.., Marco J.., Palombo C.., Kozàkovà M.., Anguissola G.B.., Cassagneau B.., Bernies M.., Distante A.., Marco I.., Fajadet J.., Zanchetti A.1998. Postischemic left ventricular dysfunction is abolished by alpha-adrenergic blocking agents. J. Am. Coll. Cardiol. 31:992–1001.
Hill J.A.., Karimi M.., Kutschke W.., Davisson R.L.., Zimmerman K.., Wang Z.., Kerber R.E.., Weiss R.M.2000. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 101:2863–2869.

Li J.., Li P.., Feng X.., Li Z.., Hou R.., Han C.., Zhang Y.2003. Effects of losartan on pressure overload-induced cardiac gene expression profiling in rats. Clin. Exp. Pharmacol. Physiol. 30:827–832.

Long C.S.., Kariya K.., Karns L.., Simpson P.C.1992. Sympathetic modulation of the cardiac myocyte phenotype: studies with a cell-culture model of myocardial hypertrophy. Basic Res. Cardiol. 87:19–31.

Mosterd A.., Hoes A.W.., de Bruyne M.C.., Deckers J.W.., Linker D.T.., Hofman A.., Grobbee D.E.1999. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur. Heart J. 20:447–455.

Murray C.J.., Lopez A.D.1997. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 349:1269–1276.

Packer M.1998. Beta-adrenergic blockade in chronic heart failure: principles, progress, and practice. Prog. Cardiovasc.
Poole-Wilson P.A.., Swedberg K.., Cleland J.G.., Di Lenarda A.., Hanrath P.., Komajda M.., Lubsen J.., Lutiger B.., Metra M.., Remme W.J.., Torp-Pedersen C.., Scherhag A.., Skene A.2003. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial Lancet. 362:7–13.
Stahl E.., Mutschler E.., Baumgartner U.., Spahn-Langguth H.1993. Carvedilol stereopharmacokinetics in rats: affinities to blood constituents and tissues. Arch. Pharm. (Weinheim). 326(9):529–533.

Figure 1.
Experimental design. Cardiac hypertrophy were confirmed at 15 weeks after abdominal aortic constriction (AC) using echocardiography. Carvedilol was dissolved in 0.5% methylcellulose and oral administered (1 mL/kg) for 3 weeks. Cardiac function, cardiac hypertrophy and electrocardiogram were performed after 18 weeks of abdominal AC.
Figure 2.
Regression of pressure overload induced cardiac hypertrophy in vivo by carvedilol. A. Representative hearts of rats treated with 18 weeks aortic constriction (AC) or 15 weeks AC+carvedilol for 3 weeks. bar=1 mm. B. Heart wet weight was normalized to body weight in each rat as an index of cardiac hypertrophy. Heart weight to body weight ratio was reversed by 2 mg/ kg/d carvedilol in hypertrophied heart induced by aortic constriction (AC). C. Liver weight to body weight ratio. R, R-carvedilol; SR, SR-carvedilol; S, S-carvedilol. †,#P<0.001 compared with sham; ∗P<0.001 compared with AC, n≥6.
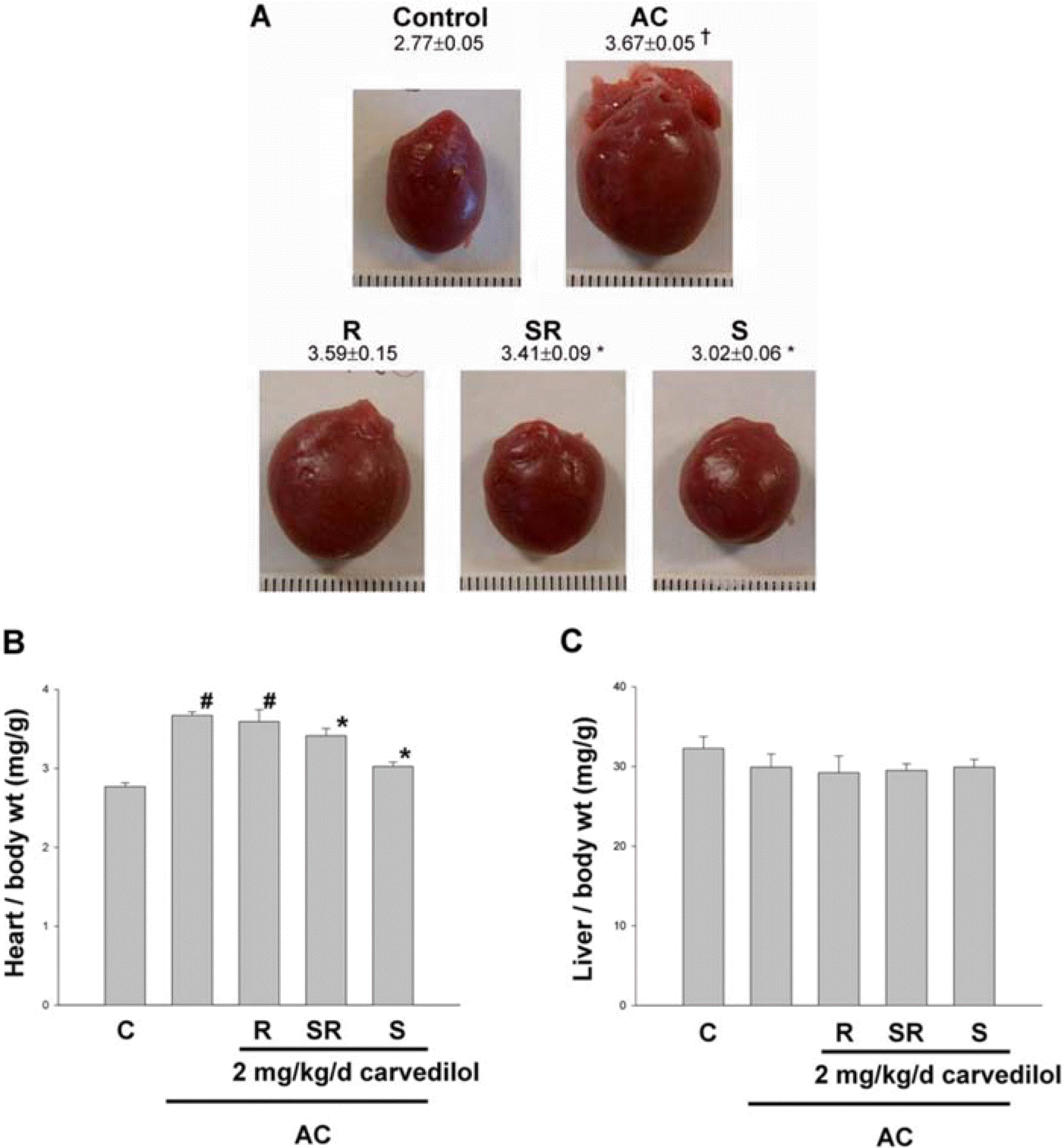
Figure 3.
Representative M-mode echocardiograms after 3 weeks of carvedilol administration in established cardiac hypertrophy induced by 15 weeks of aortic constriction (AC). Quantitative data are shown in Table 1. Concentric cardiac remodeling was observed in the AC group. The increased wall thickness was reversed by S-carvedilol. R, R-carvedilol; SR, SR-carvedilol; S, S-carvedilol.
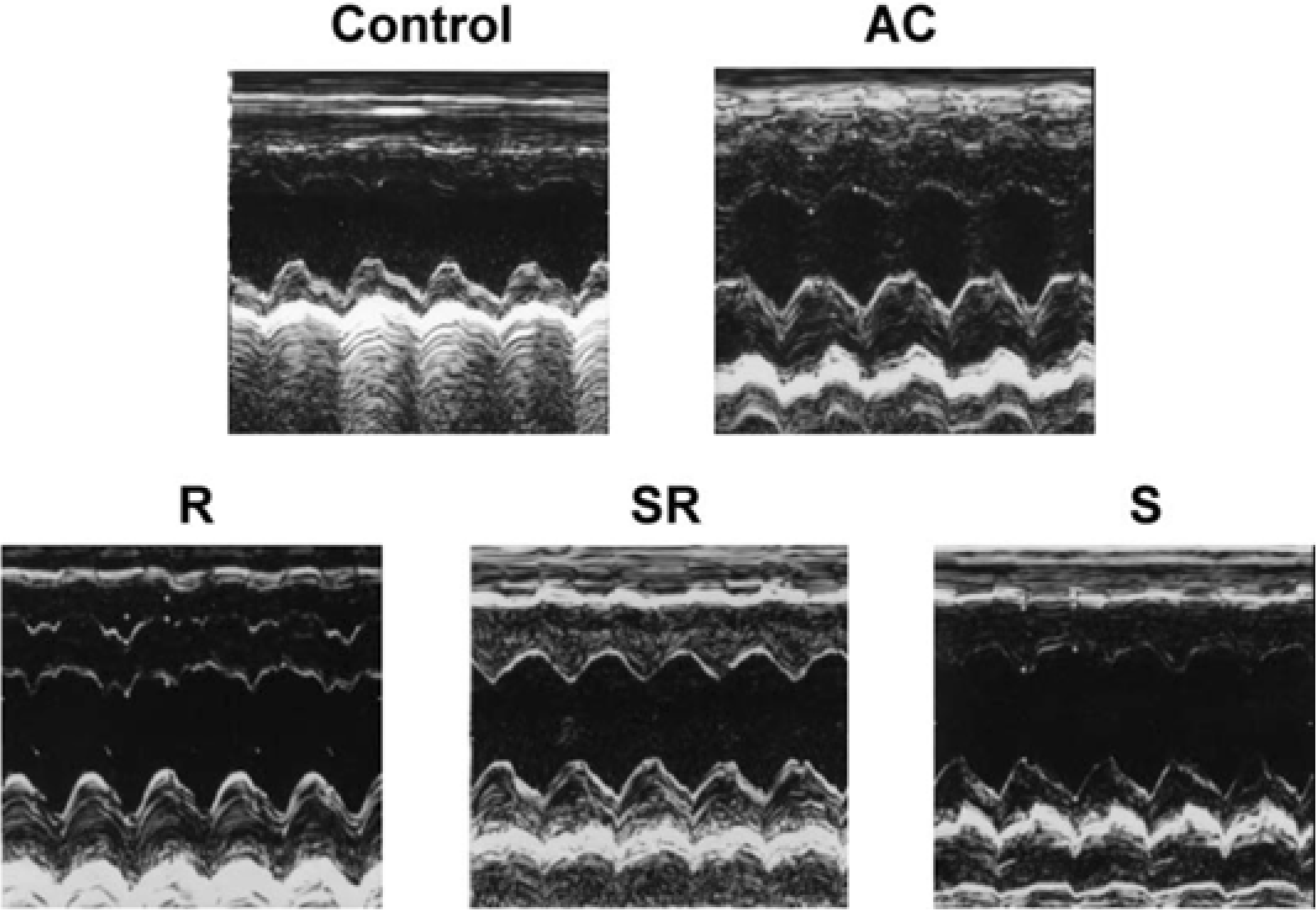
Figure 4.
Representative electrocardiograms after 3 weeks of carvedilol administration in established cardiac hypertrophy induced by 15 weeks of aortic constriction (AC). Quantitative data are shown in Table 3. Abnormal ECGs were observed in the AC group and these wave were significantly reversed by S-carvedilol. R, R-carvedilol; SR, SR-carvedilol; S, S-carvedilol.
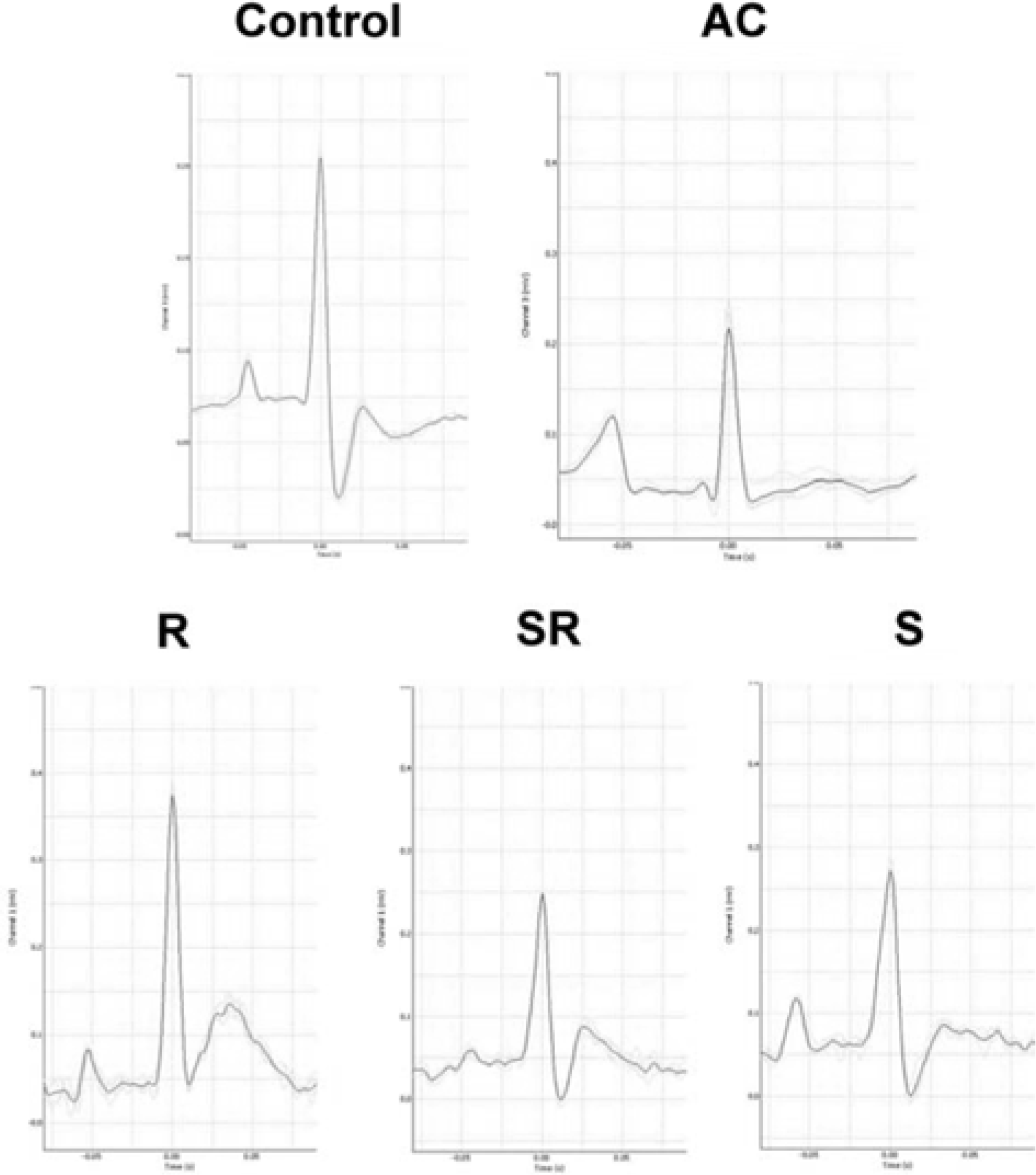
Table 1.
Echocardiographic changes after pressure overload
| 15 weeks | 18 weeks | ||||||
|---|---|---|---|---|---|---|---|
| Control | AC | Control | AC | Treatment (2 mg/kg/day) | |||
| R-Carvediol | SR-Carvediol | S-Carvediol | |||||
| IVSd (mm) | 1.22±0.04 | 1.85±0.06† | 1.75±0.05 | 2.00±0.15† | 1.73±0.12∗ | .1.78±0.06∗ | 1.70±0.09∗ |
| LVIDd (mm) | 8.22±0.50 | 7.21±0.40† | 7.82±0.60 | 7.40±0.1†0 | 7.40±0.20∗ | 7.15±0.35 | 8.53±0.13∗ |
| PWTd (mm) | 1.42±0.13 | 2.22±0.12† | 1.98±0.10 | 3.08±0.20† | 2.55±0.19∗ | .2.43±0.12∗ | 2.22±0.10∗ |
| RWT(mm/mm) | 0.34±0.01 | 0.62±0.05† | 0.51±0.01 | 0.84±0.09† | 0.68±0.06∗ | 0.67±0.08 | 0.51±0.02∗ |
| FS (%) | 30.6±2.11 | 39.72±3.850. | 43.30±3.000 | 31.2±0.40† | 37.5±3.72∗ | 33.8±5.28 | 34.4±1.69∗ |
Table 2.
The effect of carvedilol on the release of intracellular enzymes from hypertrophic heart
| AST (U/L) | ALT (U/L) | CK (U/L) | LDH (µmole/L) | |
|---|---|---|---|---|
| Control | 096.3±12.7 | 51.3±2.4 | 219.6±72.50 | 094.3±22.0 |
| AC | 134.0±28.0 | 60.0±5.0 | .524.3±177.7† | .132.7±22.6† |
| Treatment (2 mg/kg/day) | ||||
| R-Carvedilol | 445.3±312.1 | 122.0±59.5 | 652.2±177.1 | 209.0±68.2 |
| SR-Carvedilol | 144.7±23.50 | 061.3±82.1 | 775.7±256.3 | 178.3±32.1 |
| S-Carvedilol | ∗97.0±12.5∗ | ∗50.0±2.5∗ | ∗285.0±135.7∗ | 0∗83.3±21.1∗ |
Table 3.
The effect of carvedilol on ECG parameters of hypertrophic heart
| RR interval (sec) | Heart rate (bpm) | PR interval (sec) | P duration (sec) | QTc (sec) | T amplitute (mV) | |
|---|---|---|---|---|---|---|
| Control | 0.19±0.01 | 319±16 | 0.045±0.001 | 0.015±0.002 | 0.150±0.023 | .94.3±22.0 |
| AC | 0.26±0.02† | 236±18† | 0.047±0.003 | .0.019±0.001† | 0.134±0.010 | 132.7±22.6† |
| Treatment (2 mg/kg/day) | ||||||
| R-Carvedilol | 0.20±0.01∗ | 302±17∗ | 0.047±0.004 | 0.018±0.002 | 0.177±0.006 | 209.0±68.2 |
| SR-Carvedilol | 0.21±0.035∗ | 310±27∗ | 0.040±0.003 | ∗0.016±0.002∗ | 0.147±0.003 | 178.3±32.1 |
| S-Carvedilol | 0.20±0.02∗ | 318±24∗ | 0.050±0.002 | ∗0.017±0.001∗ | 0.175±0.010 | 0.83.3±21.1∗ |
AC, 18 weeks of aortic constriction; RR interval, interval from the onset of one QRS complex to the onset of the next QRS complex; PR interval, From the beginning of the P wave to the beginning of the QRS complex; QTc, QT interval corrected for rate; T wave, represents the repolarization of the ventricles.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download