Abstract
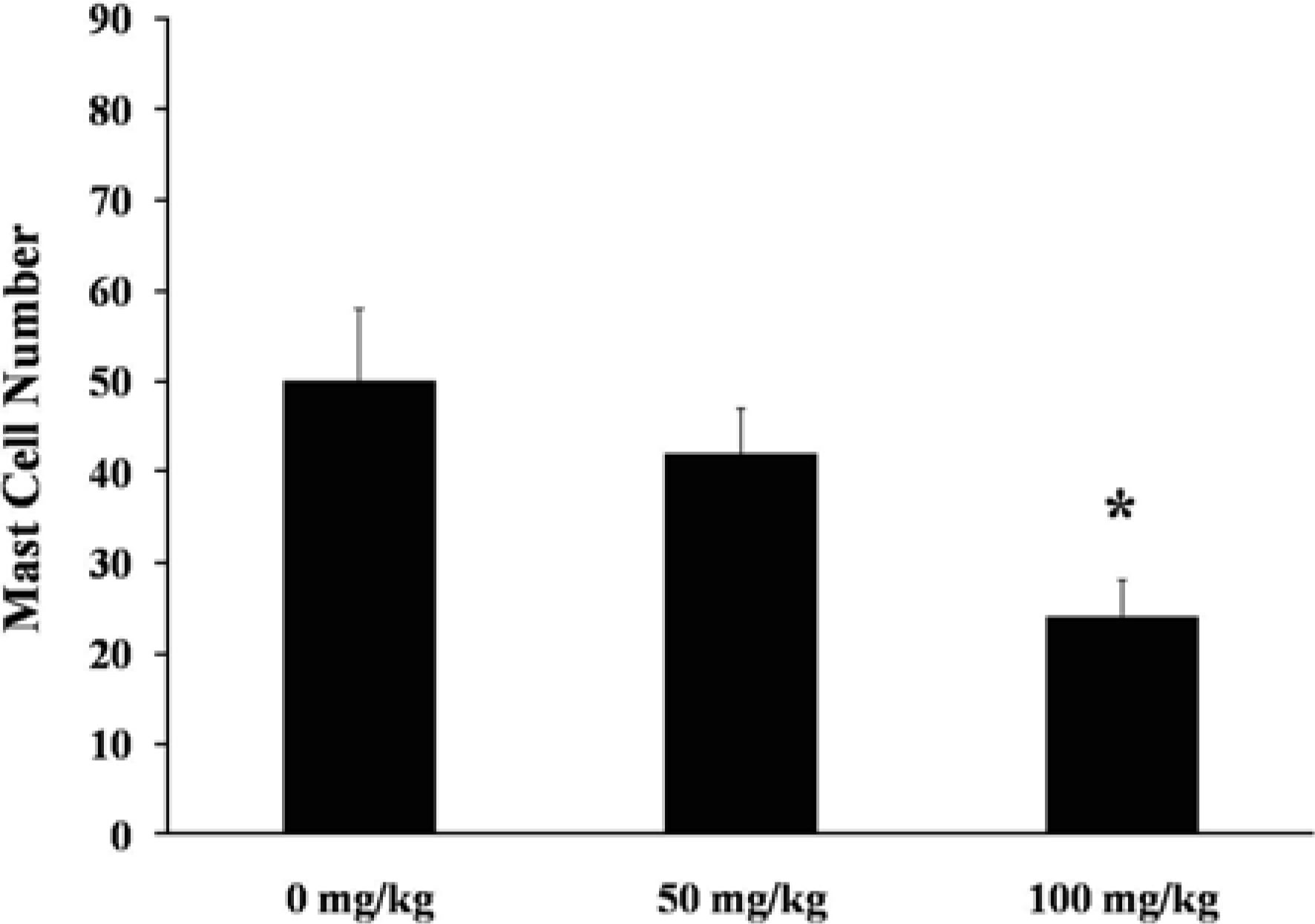
In this study, we investigated that anti-inflammatory effect of quercetin on picryl chloride(PCL)-induced contact dermatitis in BALB/c Mice. Experimental animals were divided into three groups and comprising five animals. All groups of oral administration was begun on the first day of PCL treatment and ceased on day 5. For the induction of contact dermatitis, BALB/c mice were sensitized with 40 µL of 1.5% picryl choloride (PCL) to the left and right ear, respectively. Ear swelling responses were much weaker in high-dose group (100 mg/kg) than control group (0 mg/kg). Total serum IgE levels and histamine levels were measured by sandwich ELISA method using mouse IgE, histamine measuring Kit. Both total serum IgE and histamine levels were significantly decreased in high-dose group (100 mg/kg) than other groups. Degranulation of mast cells were also confirmed by Toluidine Blue (TB) staining method. In high-dose group (100 mg/kg), the number of mast cells were significantly decreased and there are many mast cells were shown degranulation in control group (0 mg/kg). All of these results demonstrate that the pharmacological actions of quercetin indicate their potential activity for allergic inflammatory diseases through the down-regulation of mast cell activation.
REFERENCES
Acuto O.., Michel F.2003. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol.
Baadsgaard O., Wang T.1991. Immune regulation in allergic and irritant skin reactions. Int. J. Dermatol. 30, 161-Boerrigter, G.H., Bril. H. and Scheper, R.J. (1988) Hapten-specific antibodies in allergic contact dermatitis in the guinea pig. Int. Arch. Allergy Appl. Immunol. 85:385–391.

Cavallini L.., Bindoli A.., Siliprandi N.1978. Comparative evaluation of antiperoxidative action of silymarin and other flavonoids. Pharmacol. Res. Commun. 10:133–136.

Choi Y.G.., Kim S.H.., Lim J.P.., Kim D.K.., Eom D.O.., Lee K.B.., Kim S.Y.2001. Inhibitory effect of Salvia plebeia on compound 48/80-induced immediate hypersensitivity reaction. Kor. J. Pharmacogn. 32:297–301.
Cooper K.D.1994. Atopic dermatitis: recent trends in pathogenesis and therapy. J. Invest. Dermatol. 102:128–137.

Cumberbatch M.., Kimber I.1990. Phenotypic characteristics of antigen-bearing cells in the draining lymph nodes of contact sensitized mice. Immunology. 71:404–410.
Daniel R.S.., Devi K.S.., Augusti K.T.., Sudhakaran Nair C.R.2003. Mechanism of action of antiatherogenic and related effects of ficus bengalensis linn. flavonoids in experimental animals. Indian. J. Exp. Biol. 41(4):296–303.
DeKruyff R.H.., Turner T.., Abrams J.S.., Palladino MA.Jr.., Umetsu D.T.1989. Induction of human IgE synthesis by CD4+ T cell clones. Requirement for interleukin 4 and low molecular weight B cell growth factor. J. Exp. Med. 170:1477–1493.
Edenharder R.., Tang X.1997. Inhibition of the mutagenicity of 2-nitrofluorene, 3-nitrofluoranthene and 1-nitropyrene by vitamins, porphyrins and related compounds, and vegetable and fruit juices and solvent extracts. Food. Chem. Toxicol. 35(3):373–378.
Formica J.V.., Regelson W.1995. Review of the biology of quercetin and related bioflavonoids. Food. Chem. Toxicol. 33:1061–1080.

Henz B.M.., Maurer M.., Lippert U.., Worm M.., Babina M.2001. Mast cells as initiators of immunity and host defense. Exp. Dermatol. 10(1):1–10.

Hirano T.., Oka K.., Kawashima E.., Akiba M.1989. Effects of synthetic and naturally- occurring flavonoids on mitogen-induced proliferation of human peripheral-blood lymphocyt es. Life. Sci. 45:1407–1411.
Hwang S.O.., Lee K.L.2005. Identification of clcium/calmodulin-dependent phosphatase as the dephosphorylating enzyme of IgE-dependent histamine-releasing factor in RBL-2H3. Kor. J. Microbiol. Biotechnol. 33:189–193.
Ikeda M.., Kuroki K.., Suzuki H.., Nakayama H.., Saegusa J.., Doi K.2000. Picryl chloride-induced allergic dermatitis in IQI/Jic female mice. Exp. Toxicol. Pathol. 52(3):235–240.

Jung J.Y.., Saequsa J.., Nakayama H.., Doi K.2004. Comparative study on picryl chloride (PCL)-induced contact dermatitis in female IQI/Jic and BALB/c mice. Exp. Anim. 53:89–96.

Kim B.S.., Kim J.H.., Lee S.Y.., Hong S.J.2003. Influence of conventional immunotherapy with D.f and D.p on eosinophil, specific IgE, skin test reactivity, and airway hyperreactivity in house dust mite sensitive asthmatic children. Pediatr. Allergy. Respir. Dis. 13(1):8–16.
Kim H.P.., Son K.H.., Chang H.W.., Kang S.S.2004. Antiinflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 96:229–245.

Kim J.T.., Ahn S.H.., Park I.S.., Chung J.M.., Kim H.H.1998. Immunohistochemical study on the activation of cell mediated immunity in murine lymph node on allergic contact dermatitis by DNCB. DJIOM. 7:33–44.
Kim M.J.., Choi I.W.., Ahn S.C.., Shin Y.K.., Yeom S.R.., Park Y.M.2008. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol. 9:261–267.
Kimura M.., Yamada H.1984. Interaction in the antibacterial activity of flavonoids from sophora japonica L. to propionibacterium. Yakugaku. Zasshi. 104:340–346.
Lamson D.W.., Brignall M.S.2000. Antioxidants and cancer, part 3: quercetin. Altern. Med. Rev. 5(3):196–208.
Lee B.S.., Yun D.W.., Chung H.T.1992. Effects of various cytokines on the product ion of reactive oxygen intermediates murine macrophage. Kor. J. Immuol. 14(2):213–219.
Lee S.H.., Seon N. H.., Lee S.S.., Chul A.H.., Yoon H.K.., Soo P.J.., Sik P.C.2002. Positive of specific-allergen antibody and its relation in patients with allergic disease. J. Soonchunhyang. Med. Coll. 8:133–141.
Li-weber M.., Giaisi M.., Treiber M.K.., Krammer P.H.2002. The anti-inflammatory sesquiterpene lactone parthenolide suppresses IL-4 gene expression in peripheral blood T cells. Eur. J. Immunol. 32:3587–3597.

Mainardi T.., Kapoor S.., Bielory L.2009. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J. Allergy. Clin. Immunol. 123:283–294.

Metz M.., Maurer M.2007. Mast cells-key effector cells in immune responses. Trends. Immunol. 28:234–241.
Middleton E.., Mookerjee B.K.., Lee T.P.., Logue G.P.., Lippes H.A.1986. The effects of flavonoids on human lymphocyte proliferative responses. In Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological and Structure-activity Relationships., pp. 511-520, Alan R. Liss, New York.
Nakae S.., Suto H.., Iikura M.., Kakurai M.., Sedgwick J.D.., Tsai M.., Galli S.J.2006. Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 176:2238–2248.

Namgoong S.Y.., Son K.H.., Chang H.W.., Kang S.S.., Kim H.P.1994. Effects of naturally occurring flavonoids on mitogen-induced lymphocyte proliferation and mixed lymphocyte culture. Life Sci. 54:313–320.

Park D.S.., Kim H.H.., Lee J.S.1998. The significance of family history, Immunoglobulin E, total eosinophils and eosinophil cationic protein as a predictor of allergic diseases. J. Korean. Pediatr. Soc. 41:1273–1282.
Park H.H.., Lee S.Y.., Son H.Y.., Park S.B.., Kim M.S.., Choi E.J.., Singh T.S.., Ha J.H.., Lee M.G.., Kim J.E.., Hyun M.C.., Kwon T.K.., Kim Y.H.., Kim S.H.2008. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 31:1303–1311.

Park H.J.., Lee C.M.., Jung I.D.., Lee J.S.., Jeong Y.I.., Chang J.H.., Chun S.H.., Kim M.J.., Kim B.S.., Park I.S.., Pyun B.Y.2001. Comparative study for detection of specific IgE in allergic disease; skin prick test, RAST, and dipstick test. Pediatr. Allergy. Respir. Dis. 11:233–239.
Park M.C.., Kim K.J.., Lee H.S.., Jo E.H.2007. Attenuation of airway hyperreactivity (AHR) and inflammation by water extract of Rubus coreanus MIQ (WRCM). Korean. Oriental. Medical. Ophthalmology & Otolaryngology & Dermatology.
Park S.., Jung S.H.., An E.N.2006. The effect of exercise preconditioning and quercetin on animal model of ischemic brain damage. Korean. J. Exercise. Nutrition. 10(3):315–321.
Peden D.B.., Dailey L.1995. Modulation of mast cell functions by in vitro ozone expos ure. Am. J. Physiol. 4:902–910.
Rees J.L.., Friedmann P.S.., Matthews J.N.1990. The influence of area of application on sensitization by dinitrochlorobenzene. Br. J. Dermatol. 122:29–31.

Suh K.S.., Kwon M.S.., Cho J.S.2004. Anti-anaphylactic effects of natural extract compound(AllerQ) in the rats. J. East. Asian. Soc. Dietary. Life. 14(5):425–437.
Figure 1.
Ear swelling responses in BALB/c mice at 0, 3, 6, 9, 12, 18, 24 hr following the sensitization with PCL to the ear. Each value was expressed as Mean±SE of 5 BALB/c mice. ∗Significantly different from 0 mg/kg group at P<0.05.
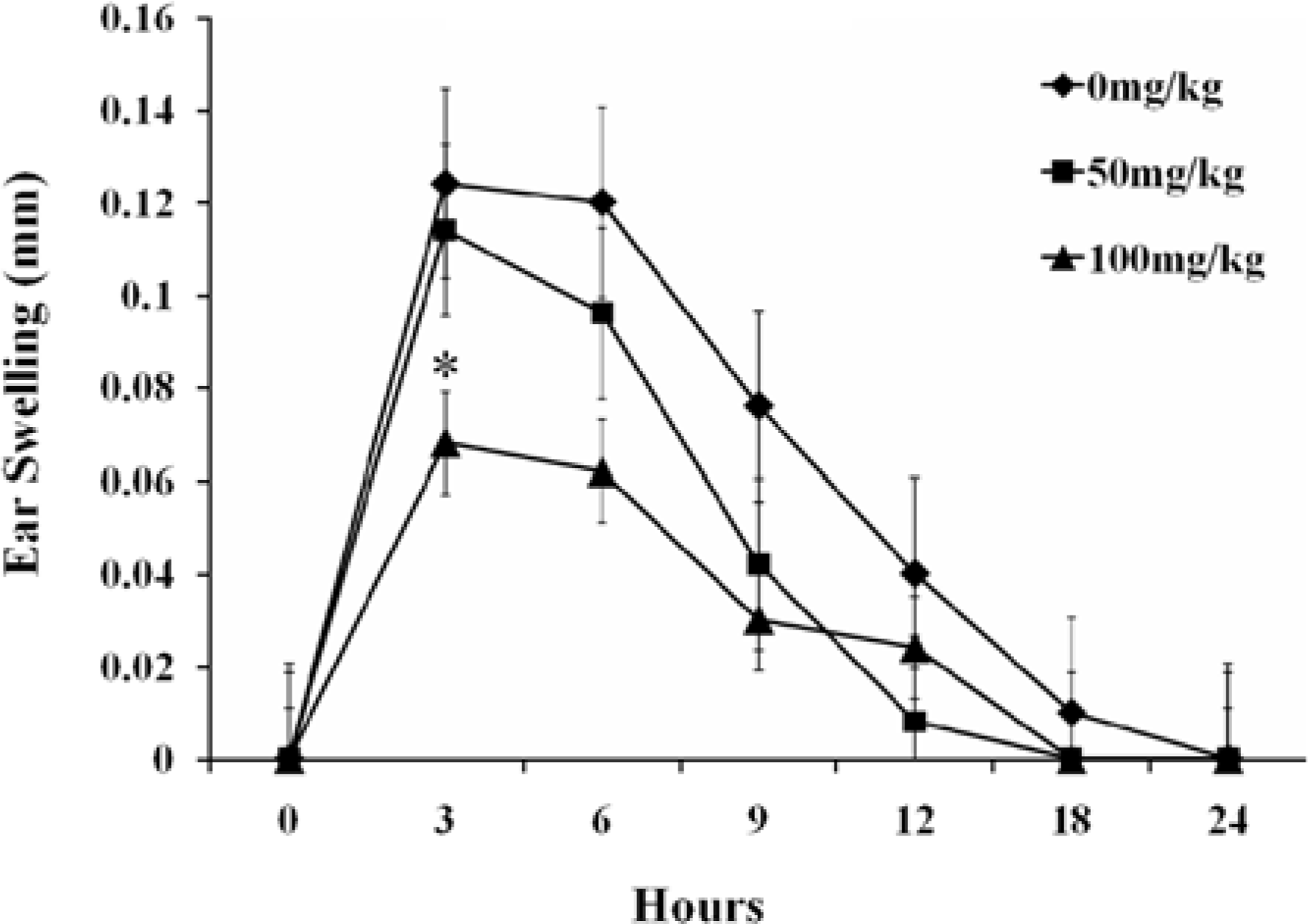
Figure 2.
Total serum IgE levels in female BALB/c mice at 3 hr after application with PCL. Each value was expressed as Mean±SE of 5 BALB/c mice. ∗Significantly different from 0 mg/ kg group at P<0.05.
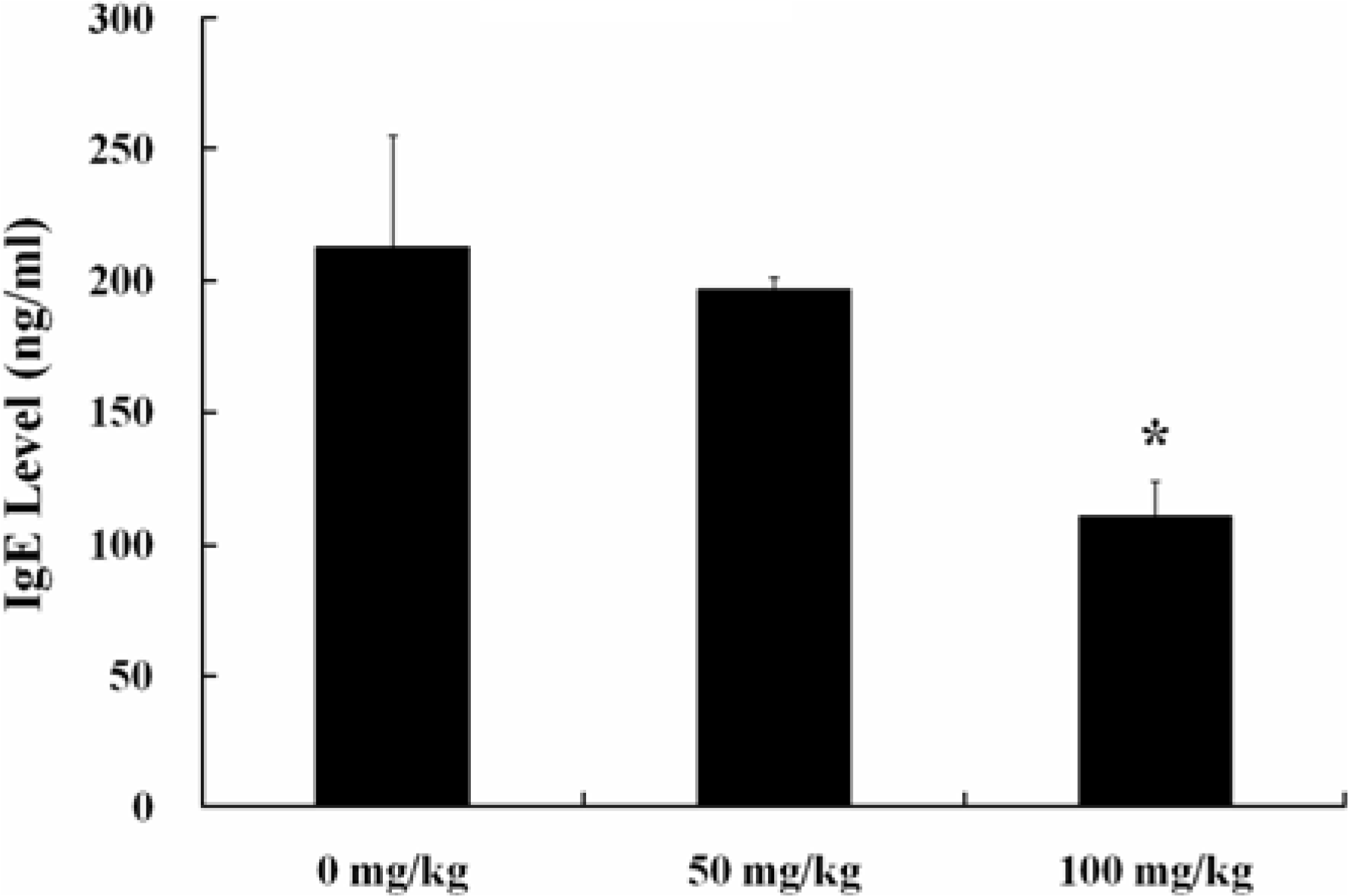
Figure 3.
Total serum Histamine levels in female BALB/c mice at 3 hr after application with PCL. Each value was expressed as Mean±SE of 5 BALB/c mice. ∗Significantly different from 0 mg/ kg group at P<0.05.
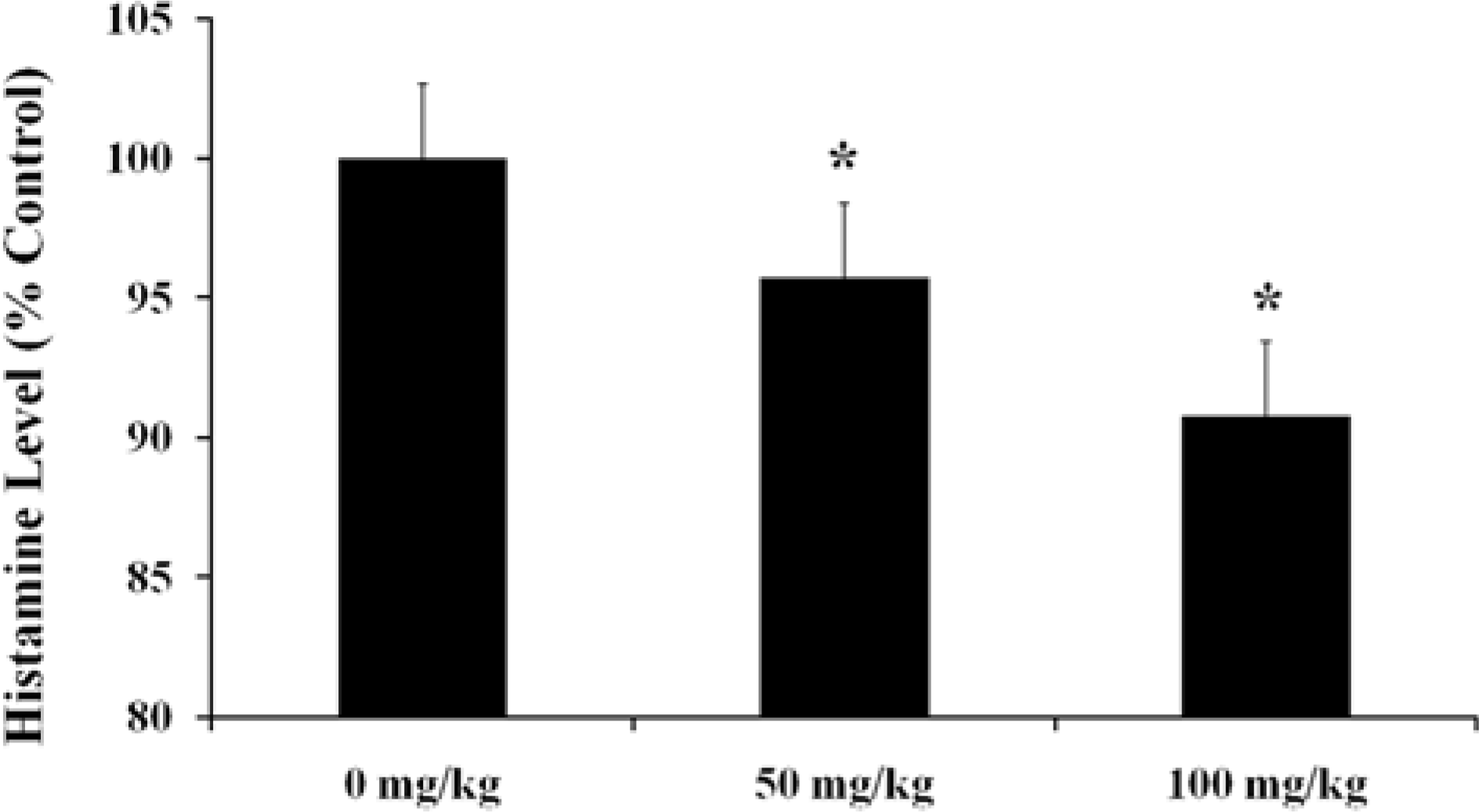
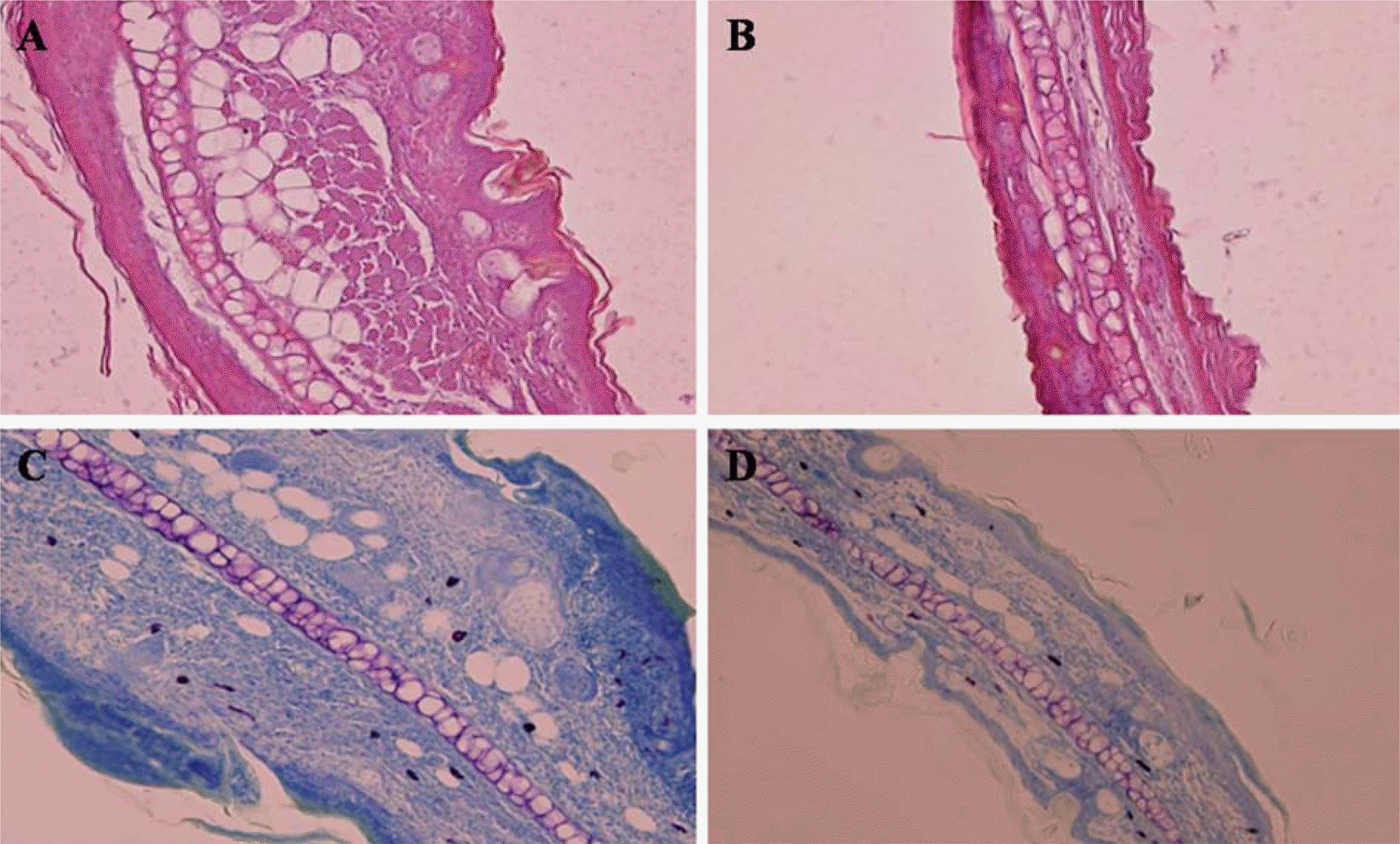
Figure 4.
Ear skin of 0 mg/kg group (A, C) and 100 mg/kg quercetin group (B, D) in BALB/c mice at 3 hr after application with PCL. (A) Intradermal edema with prominent inflammatory cell infiltration. H&E, ×200. (B) Less severe changes are seen compared with (A). H&E, ×200. (C) There are many mast cells including those showing degranulation. TB ×200. (D) Mast cells show less degranulation compared with (C). TB ×200.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download