Falus A.., Merétey K.1992. Histamine: an early messenger in inflammatory and immune reactions. Immunol. Today. 13(5):154–156.
Friedman P.S.2006. Contact sensitization and allergic contact dermatitis immunobiological mechanisms. Toxicol. Lett. 162:49–54.
Furue M.., Terao H.., Moroi Y.., Koga T.., Kubota Y.., Nakayama J.., Furukawa F.., Tanaka Y.., Katayama I.., Kinukawa N.., Nose Y.., Urabe K.2004. Dosage and adverse effects of topical tacrolimus and steroids in daily management of atopic dermatitis. J. Dermatol. 31(4):277–283.

Goutet M.., Pépin E.., Langonné I.., Huguet N.., Ban M.2005. Identification of contact and respiratory sensitizers using flow cytometry. Toxicol. Appl. Pharmacol. 205(3):259–270.

Hruza L.L.., Pentland A.P.1993. Mechanism of UV induced inflammation. J. Invest. Dermatol. 15(2):111–115.
Kaefer C.M.., Milner J.A.2008. The role of herbs and spices in cancer prevention. J. Nutri. Biochem. 19:347–361.

Lee S.H.., Baek S.J.., Kim H.A.., Heo Y.2006. 2,4-dinitrochlorobenzene-induced atopic dermatitis like immune alteration in mice. J. Toxicol. Pub. Health. 22(4):357–364.
Malaviya R.., Morrison A.R.., Pentland A.P.1996. Histamine in human epidermal cells is induced by ultraviolet light injury. J. Invest. Dermatol. 106(4):785–789.

Matsuda H.., Watanabe N.., Geba G.P.., Sperl J.., Tsudzuki M.., Hiroi J.., Matsumoto M.., Ushio H.., Saito S.., Askenase P.W.., Ra C.1997. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 9(3):461–466.

Matsumoto M.., Ra C.., Kawamoto K.., Sato H.., Itakura A.., Sawada J.., Ushio H.., Suto H.., Mitsuishi K.., Hikasa Y.., Matsuda H.1999. IgE hyperproduction through enhanced tyrosine phosphorylation of Janus kinase 3 in NC/Nga mice, a model for human atopic dermatitis. J. Immunol. 162(2):1056–1063.
McGirt L.Y.., Beck L.A.2006. Innate immune defects in atopic dermatitis. J. of Allergy and Clin. Immunol. 118(1):202–208.

Menzies-Gow A.., Ying S.., Sabroe I.., Stubbs V.L.., Soler D.., Williams T.J.., Kay A.B.2002. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J. Immunol. 169(5):2712–2718.

Mueller D.L.., Jenkins M.K.., Schwartz R.H.1989. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol. 7:445–480.

Plitnick L.M.., Loveless S.E.., Ladics G.S.., Holsapple M.P.., Selgrade M.J.., Sailstad D.M.., Smialowicz R.J.2002. Cytokine profiling for chemical sensitizers: application of the ribonuclease protection assay and effect of dose. Toxicol. Appl. Pharmacol. 179(3):145–154.

Renz H.., Brodie C.., Bradley K.., Leung D.Y.., Gelfand E.W.1994. Enhancement of IgE production by anti-CD40 antibody in atopic dermatitis. J. Allergy Clin. Immunol. 93(3):658–668.

Schuller E.., Oppel T.., Bornhövd E.., Wetzel S.., Wollenberg A. 2004. Tacrolimus ointment causes inflammatory dendritic epidermal cell depletion but no Langerhans cell apoptosis in patients with atopic dermatitis. J. Allergy and Clin. Immunol. 114(1):137–143.

Schultz-Larsen F.., Hanifin J.M.2002. Epidemiology of atopic dermatitis. Immunol. Allergy Clin. North Am. 22:1–24.
Shiohara T.., Hayakawa J.., Mizukawa Y.2004. Animal models for atopic dermatitis: are they relevant to human disease? J. Dermatol. Sci. 36(1):1–9.

Taniguchi Y.., Kohno K.., Inoue S.., Koya-Miyata S.., Okamoto I.., Arai N.., Iwaki K.., Ikeda M.., Kurimoto M.2003. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice.
Touitou E.., Dayan N.., Bergelson L.., Godin B.., Eliaz M.2000. Ethosomes-novel vesicular carriers for enhanced delivery: characterization and skin penetration properties J. Control. Release. 65:403–418.
Touitou E.., Meidan V.M.., Horwitz E.1998. Methods for quantitative determination of drug localized in the skin. J. Control. Release. 56:7–21.

Wisselink M.A.., Willemse T. 2009. The efficacy of cyclosporine A in cats with presumed atopic dermatitis: A double blind, randomised prednisolone-controlled study. The Vet. Journal. 180:55–59.

Yano S.., Umeda D.., Yamashita S.., Yamada K.., Tachibana H.2009. Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Nutri. Biochem. 20(11):876–881.

Ying S.., Robinson D.S.., Meng Q.., Barata L.T.., McEuen A.R.., Buckley M.G.., Walls A.F.., Askenase P.W.., Kay A.B.1999. C-C chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-hour eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-hour tissue eosinophilia, and relationship to basophils and other C-C chemokines (monocyte chemoattractant protein-3 and RANTES). J. Immunol. 163(7):3976–3984.
Yun J.W.., Kim H.., Kang H.J.., Koh J.Y.., Kim B.H.2008. Models for Atopic Dermatitis in NC/Nga Mice. Lab. Animal Research. 24(1):59–66.
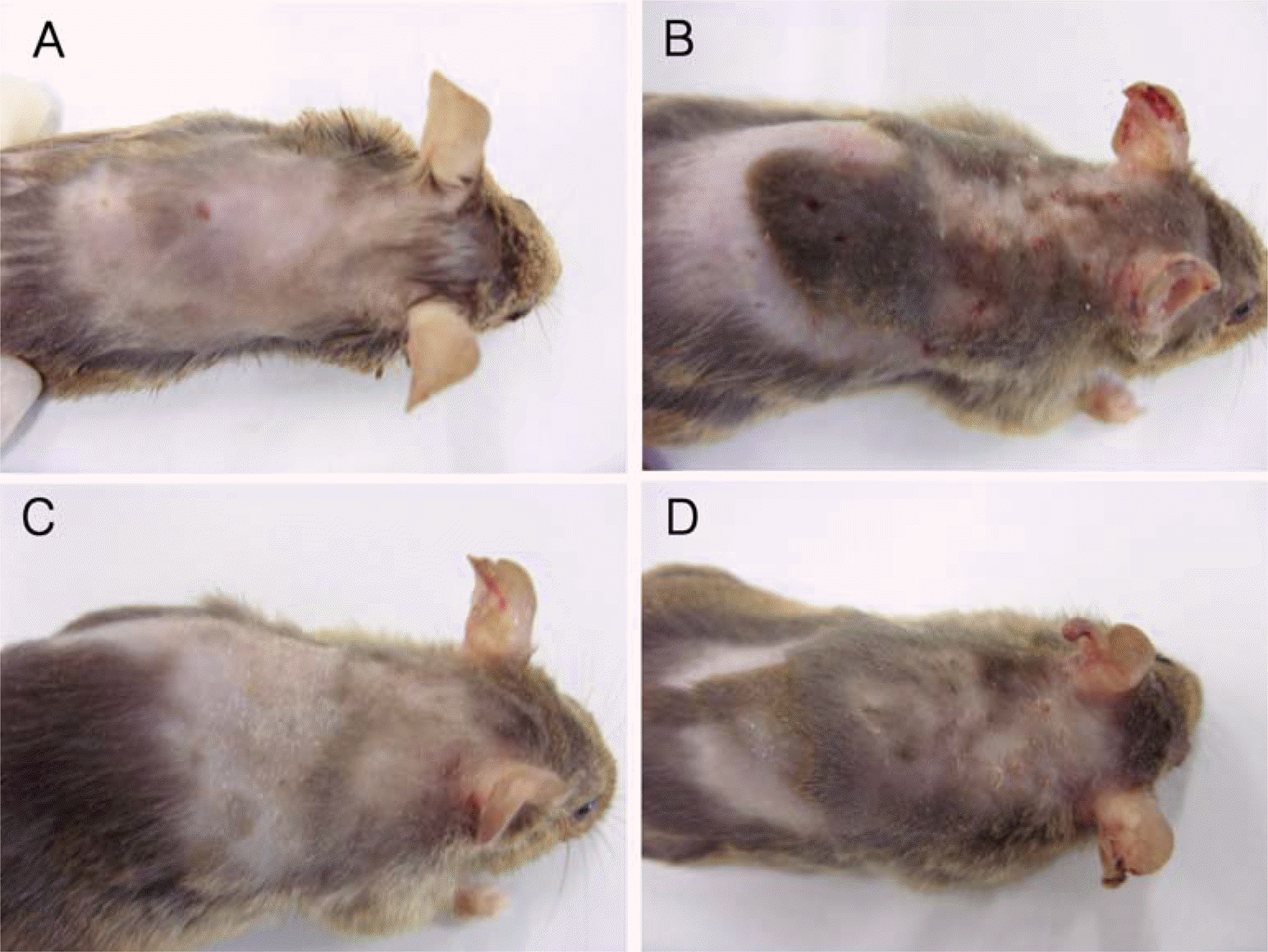
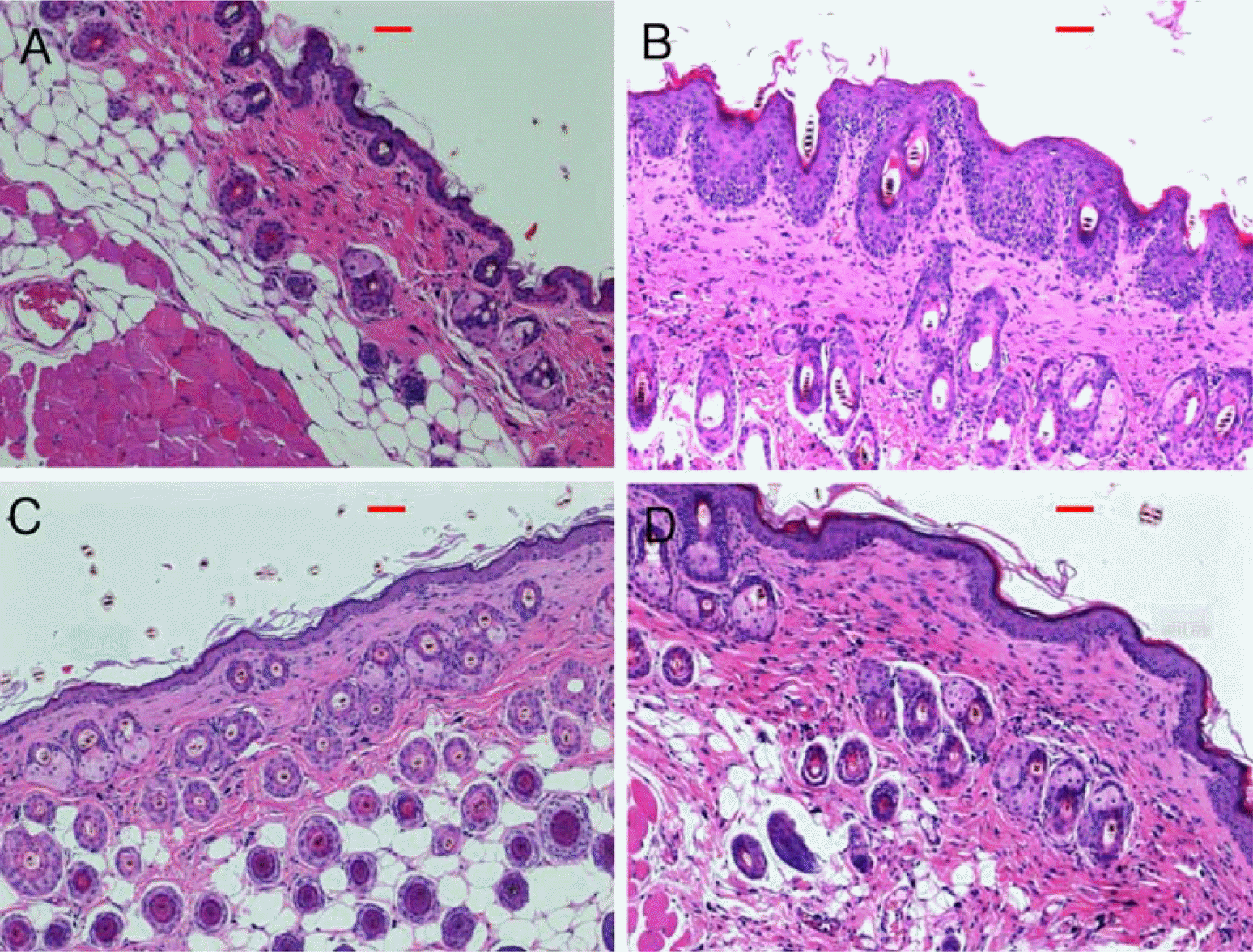
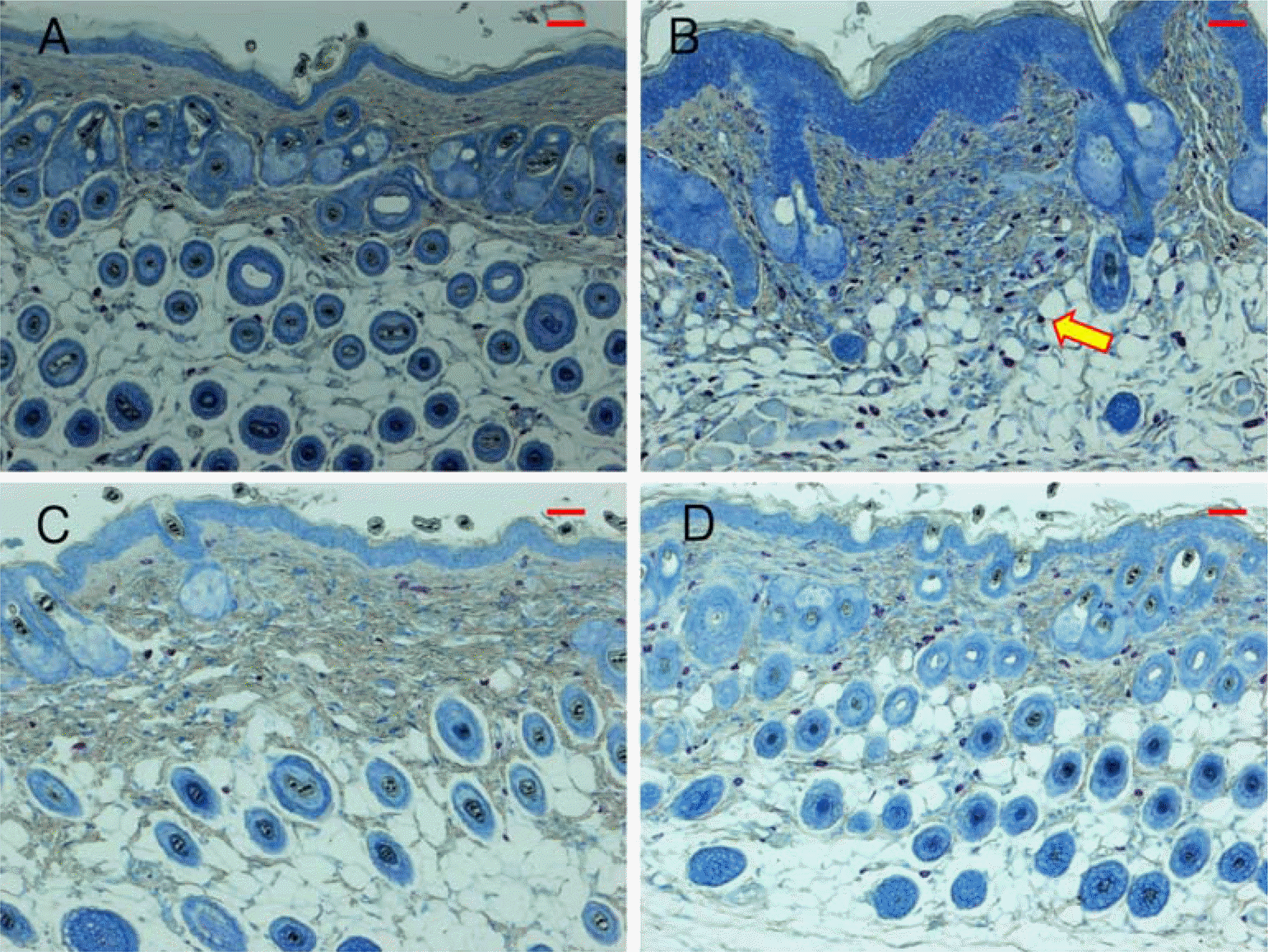




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download